Abstract
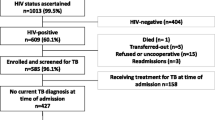
Extra-pulmonary tuberculosis (EPTB) continues to be difficult to diagnose. Novel biomarkers in biological specimens offer promise. Detection of Mycobacterium tuberculosis (Mtb) DNA in urine could prove useful in diagnosis of EPTB, possibly due to disseminated disease or micro-abscesses reported in kidneys. The current study was designed to detect Mtb DNA in stored urine samples from patients with EPTB. Diagnosis of EPTB was reached using Microbiological Reference Standards (MRS) on samples from the disease site using WHO Recommended Diagnostics (WRD), [smear microscopy, liquid culture (MGIT-960)] and GX (molecular WRD, mWRD) and Comprehensive reference standards [CRS, clinical presentation, microbiological reference standards, radiology, histopathology]. GX-Ultra was performed on urine samples stored in -80oC deep freezer, retrospectively. Of 70 patients, 51 (72.9%) were classified as confirmed TB, 11 (15.7%) unconfirmed TB, and 8 (11.4%) unlikely TB. GX-Ultra in urine samples demonstrated sensitivity of 52.9% and specificity of 57.9% against MRS, and higher sensitivity of 56.5% and specificity of 100% against CRS. The sensitivity and specificity of GX-Ultra in urine was 53.6% and 75% for pus sample subset and 52.2% and 53.3% for fluid sample subset. Urine being non-invasive and easy to collect, detection of Mtb DNA using mWRD in urine samples is promising for diagnosis of EPTB.
Similar content being viewed by others
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
G. W. H. Organization;, “Global tuberculosis report,” (2021)
Sharma SK, Mohan A (2004) “Extrapulmonary tuberculosis.,” Indian J. Med. Res, vol. 120, no. 4, pp. 316–53, Oct. [Online]. Available: http://www.ncbi.nlm.nih.gov/pubmed/15520485
Lee JY (2015) Diagnosis and treatment of Extrapulmonary Tuberculosis. Tuberc Respir Dis (Seoul) 78(2):47. https://doi.org/10.4046/trd.2015.78.2.47
Lawn SD, Gupta-Wright A (2016) “Detection of lipoarabinomannan (LAM) in urine is indicative of disseminated TB with renal involvement in patients living with HIV and advanced immunodeficiency: evidence and implications,” Trans. R. Soc. Trop. Med. Hyg, vol. 110, no. 3, pp. 180–185, Mar. https://doi.org/10.1093/trstmh/trw008
Labugger I et al (Jun. 2017) Detection of transrenal DNA for the diagnosis of pulmonary tuberculosis and treatment monitoring. ” Infect 45(3):269–276. https://doi.org/10.1007/s15010-016-0955-2
Cannas A et al (2008) “Mycobacterium tuberculosis DNA detection in soluble fraction of urine from pulmonary tuberculosis patients.,” Int. J. Tuberc. Lung Dis, vol. 12, no. 2, pp. 146–51, [Online]. Available: http://www.ncbi.nlm.nih.gov/pubmed/18230246
Wu X et al (2019) “Assessment of the Xpert MTB/RIF Ultra assay on rapid diagnosis of extrapulmonary tuberculosis,” Int. J. Infect. Dis, vol. 81, pp. 91–96, https://doi.org/10.1016/j.ijid.2019.01.050
Sabi I et al (2022) Diagnosis of paediatric TB using Xpert ® MTB/RIF Ultra on fresh respiratory samples. Int J Tuberc Lung Dis 26(9):862–868. https://doi.org/10.5588/ijtld.22.0007
Pang Y et al (Jul. 2017) GeneXpert MTB/RIF assay in the diagnosis of urinary tuberculosis from urine specimens. ” Sci Rep 7(1):6181. https://doi.org/10.1038/s41598-017-06517-0
Graham SM et al (2015) “Clinical Case Definitions for Classification of Intrathoracic Tuberculosis in Children: An Update.,” Clin. Infect. Dis, vol. 61Suppl 3, pp. S179-87, https://doi.org/10.1093/cid/civ581
Kent E, Kubica PT (1985) GP, Public health mycobacteriology: a guide for the Level III laboratory. Centers for Disease Control, Atlanta, GA
Forbes BA et al (Apr. 2018) Practical Guidance for Clinical Microbiology Laboratories: Mycobacteria. Clin Microbiol Rev 31(2). https://doi.org/10.1128/CMR.00038-17
Mechal Y et al (Dec. 2019) Evaluation of GeneXpert MTB/RIF system performances in the diagnosis of extrapulmonary tuberculosis. BMC Infect Dis 19(1):1069. https://doi.org/10.1186/s12879-019-4687-7
Singh UB et al (2016) Genotypic, phenotypic and clinical validation of GeneXpert in Extra-Pulmonary and Pulmonary Tuberculosis in India. ” PLoS One 11(2):e0149258. https://doi.org/10.1371/journal.pone.0149258
Tuuminen T (2012) Urine as a Specimen to diagnose infections in Twenty-First Century: Focus on Analytical Accuracy. Front Immunol 3. https://doi.org/10.3389/fimmu.2012.00045
Atherton RR et al (Oct. 2018) Detection of Mycobacterium tuberculosis in urine by Xpert MTB/RIF Ultra: a useful adjunctive diagnostic tool in HIV-associated tuberculosis. Int J Infect Dis 75:92–94. https://doi.org/10.1016/j.ijid.2018.07.007
Yang X et al (2020) “Cell-free Mycobacterium tuberculosis DNA test in pleural effusion for tuberculous pleurisy: a diagnostic accuracy study,” Clin. Microbiol. Infect, vol. 26, no. 8, pp. 1089.e1-1089.e6, https://doi.org/10.1016/j.cmi.2019.11.026
Park JH et al (2022) “Utility of plasma cell-free DNA detection using homobifunctional imidoesters using a microfluidic system for diagnosing active tuberculosis,” Infect. Dis. (Auckl), vol. 54, no. 1, pp. 46–52, https://doi.org/10.1080/23744235.2021.1963839
Fernández-Carballo BL, Broger T, Wyss R, Banaei N, Denkinger CM (Apr. 2019) Toward the development of a circulating free DNA-Based in Vitro Diagnostic Test for Infectious Diseases: a review of evidence for tuberculosis. J Clin Microbiol 57(4). https://doi.org/10.1128/JCM.01234-18
Click ES et al (Jan. 2018) Detection of apparent cell-free M. tuberculosis DNA from plasma. ” Sci Rep 8(1):645. https://doi.org/10.1038/s41598-017-17683-6
Pan S-W et al (Nov. 2022) Circulating mitochondrial cell-free DNA dynamics in patients with mycobacterial pulmonary infections: potential for a novel biomarker of disease. Front Immunol 13. https://doi.org/10.3389/fimmu.2022.1040947
Petrucci R et al (Jan. 2015) Use of Transrenal DNA for the diagnosis of Extrapulmonary Tuberculosis in Children: a case of Tubercular Otitis Media. J Clin Microbiol 53(1):336–338. https://doi.org/10.1128/JCM.02548-14
Gopalaswamy R, Dusthackeer VNA, Kannayan S, Subbian S (May 2021) Extrapulmonary Tuberculosis—An update on the diagnosis, treatment and drug resistance. J Respir 1(2):141–164. https://doi.org/10.3390/jor1020015
Hoel IM, Syre H, Skarstein I, Mustafa T (2020) Xpert MTB/RIF ultra for rapid diagnosis of extrapulmonary tuberculosis in a high-income low-tuberculosis prevalence setting. Sci Rep 10(1):1–7. https://doi.org/10.1038/s41598-020-70613-x
Zhang M, Xue M, qing He J (2020) Diagnostic accuracy of the new Xpert MTB/RIF Ultra for tuberculosis disease: a preliminary systematic review and meta-analysis. Int Soc Infect Dis 90. https://doi.org/10.1016/j.ijid.2019.09.016
Green C, Huggett JF, Talbot E, Mwaba P, Reither K, Zumla AI (Aug. 2009) Rapid diagnosis of tuberculosis through the detection of mycobacterial DNA in urine by nucleic acid amplification methods. ” Lancet Infect Dis 9(8):505–511. https://doi.org/10.1016/S1473-3099(09)70149-5
“Circulating Tumor DNA Guiding Adjuvant Therapy in Colon Cancer,” N. Engl. J. Med., vol. 387, no. 8, pp. 759–760 (2022) https://doi.org/10.1056/NEJMc2209374
Ssengooba W et al (2020) Accuracy of Xpert Ultra in diagnosis of pulmonary tuberculosis among children in Uganda: a Substudy from the SHINE Trial. J Clin Microbiol 58(9). https://doi.org/10.1128/JCM.00410-20
Acknowledgements
The work reported was the analysis of patient diagnostic tests, carried out for patient care. We would like to acknowledge technical help from Ms. Megha (Junior Research Fellow).
Funding
This work was supported by the All India Institute of Medical Sciences, New Delhi, and FIND, the global alliance for diagnostics, Geneva.
Author information
Authors and Affiliations
Contributions
UBS conceived the idea for the research and planned the work. UBS and AKP performed the literature search and wrote the article. UBS, RL, PN, and MR contributed to diagnostics and formal analysis. KS and RB contributed to laboratory management. UBS and RLcontributed to the study design, clinical discussion, and analysis. AB, SKK, SS, and RL contributed to clinical data and clinical care.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Ethical Approval
Institutional ethical clearance from AIIMS, New Delhi, India, was obtained for the study (IEC-5/09.02.2017).
Consent to Participate
The procedures followed in the study were in accordance with the ethical standards of the Helsinki declaration (1964, amended most recently in 2008) of the World Medical Association. Written informed consent was obtained from all the patients or parents/guardians before inclusion in the study.
Consent to Publish
Patients or parents/guardians signed informed consent regarding publishing their data.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
284_2023_3503_MOESM1_ESM.pdf
Supplementary Table S1: Summary of results from EPTB samples used in the study.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Singh, U.B., Angitha, K.P., Bhatnagar, A. et al. GeneXpert Ultra in Urine Samples for Diagnosis of Extra-Pulmonary Tuberculosis. Curr Microbiol 80, 361 (2023). https://doi.org/10.1007/s00284-023-03503-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00284-023-03503-w