Abstract
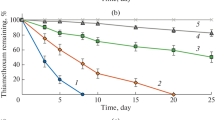
Thiobencarb has been extensively applied for weed control, resulting in severe environmental problems. In this study, thiobencarb degradation in liquid media and in soil by two bacterial strains, Pseudomonas sp. Th1 and Cupriavidus oxalaticus Th2, was investigated. Both bacterial isolates utilized the compound as a sole carbon, nitrogen and sulfur source. The utilization rates of thiobencarb by Pseudomonas sp. Th1 and C. oxalaticus Th2 in a liquid mineral medium were 1.02 ± 0.11 and 0.80 ± 0.07 µM/h at 100 µM, respectively. The determination of degradation and bacterial growth rates kinetics showed that the rates for pure thiobencarb followed the Michaelis–Menten model; meanwhile, the rates for thiobencarb in a commercial herbicide fitted well with the Edwards model. Their degradation by the mixed culture of both strains reduced the accumulation of intermediate products, including S-4-chlorobenzyl ethylthiocarbamate and 4-chlorobenzyl mercaptan, in media. The degradation by the mixed culture of these bacteria immobilized in rice straw was significantly higher than those of their free counterparts when determining in a packed bed bioreactor (P < 0.05). In addition, the inoculation of the mixed bacterial culture in soil significantly enhanced the degradation performance for both thiobencarb and propanil in a commercial herbicide. This study elucidates the differences in biodegradation of pure thiobencarb and thiobencarb in an herbicide.





Similar content being viewed by others
References
Fernández-Vega C, Sancho E, Ferrando MD, Andreu E (2002) Thiobencarb-induced changes in acetylcholinesterase activity of the fish Anguilla anguilla. Pesti Biochem Physiol 72:55–63. https://doi.org/10.1006/pest.2001.2581
Jena PK, Adhya TK, Rao VR (1990) Nitrogen-fixing bacterial populations as influenced by butachlor and thiobencarb in rice soils. Zentralbl Mikrobiol 145:469–474. https://doi.org/10.1016/S0232-4393(11)80165-4
EPA (1997) Thiobencarb. R.E.D. FACTS, United States Environmental Protection Agency. EPA-738-F-97-013. https://www3.epa.gov/pesticides/chem_search/reg_actions/reregistration/fs_PC-108401_1-Sep-97.pdf
Sapari P, Ismail BS (2012) Pollution levels of thiobencarb, propanil, and pretilachlor in rice fields of the muda irrigation scheme, Kedah, Malaysia. Environ Monit Assess 184:6347–6356. https://doi.org/10.1007/s10661-011-2424-9
Amin MN, Kaneco S, Kato T, Katsumata H, Suzuki T, Ohta K (2008) Removal of thiobencarb in aqueous solution by zero valent iron. Chemosphere 70:511–515. https://doi.org/10.1016/j.chemosphere.2007.09.017
Palumbo AJ, Tenbrook PL, Phipps A, Tjeerdema RS (2004) Comparative toxicity of thiobencarb and deschlorothiobencarb to rice (Oryza sativa). Bull Environ Contam Toxicol 73:213–218. https://doi.org/10.1007/s00128-004-0415-z
Lai H-F, Chen C-C, Chang Y-K, Lu C-S, Wu R-J (2014) Efficient photocatalytic degradation of thiobencarb over BiVO4 driven by visible light: parameter and reaction pathway investigations. Sep Purif Technol 122:78–86. https://doi.org/10.1016/j.seppur.2013.10.049
Huang S, Chen C, Tsai H, Shaya J, Lu C (2018) Photocatalytic degradation of thiobencarb by a visible light-driven MoS2 photocatalyst. Sep Purif Technol 197:147–155. https://doi.org/10.1016/j.seppur.2018.01.009
Nakamura Y, Ishikawa K, Kuwatsuka S (1977) Degradation of benthiocarb in soils as affected by soil conditions. J Pestic Sci 2:7–16. https://doi.org/10.1584/jpestics.2.7
Miwa N, Takeda Y, Kuwatsuka S (1988) Plasmid in the degrader of the herbicide thiobencarb (benthiocarb) isolated from soil: a possible mechanism for enrichment of pesticide degraders in soil. J Pestic Sci 13:291–293. https://doi.org/10.1584/jpestics.13.291
Torra-Reventós M, Yajima M, Yamanaka S, Kodama T (2004) Degradation of the herbicides thiobencarb, butachlor and molinate by a newly isolated Aspergillus niger. J Pestic Sci 29:214–216. https://doi.org/10.1584/jpestics.29.214
Chu CW, Liu B, Li N, Yao SG, Cheng D, Zhao JD, Qiu JG, Yan X, He Q, He J (2017) A novel aerobic degradation pathway for thiobencarb is initiated by the TmoAB two-component flavin mononucleotide-dependent monooxygenase system in Acidovorax sp. strain T1. Appl Environ Microbiol 83:e01490-e1517. https://doi.org/10.1128/AEM.01490-17
Ferrando MD, Alarcon V, Fernandez-Casalderrey A, Gamón M, Andreu-Moliner E (1992) Persistence of some pesticides in the aquatic environment. Bul Environ Toxicol 48:747–755. https://doi.org/10.1016/0043-1354(76)90035-X
Quayle WC, Oliver DP, Zrna S (2006) Field dissipation and environmental hazard assessment of clomazone, molinate, and thiobencarb in Australian rice culture. J Agric Food Chem 54:213–220. https://doi.org/10.1021/jf061107u
Braverman MP, Locascio SJ, Dusky JA, Hornsby AG (1990) Mobility and bioactivity of thiobencarb. Weed Sci 38:607–614. https://doi.org/10.1017/S0043174500051572
Mahmoudi MR, Rahnemaie A, Es-haghi MMJ (2013) Kinetics of degradation and adsorption–desorption isotherms of thiobencarb and oxadiargyl in calcareous paddy fields. Chemosphere 91(7):1009–1017. https://doi.org/10.1016/j.chemosphere.2013.01.077
Saison C, Waller NJ, Kumar A, Kookana RS (2009) Effects of thiobencarb in combinations with molinate and chlorpyrifos on selected soil microbial processes. J Environ Sci Health B 44(3):226–234. https://doi.org/10.1080/03601230902728195
Edwards VH (1970) The influence of high substrate concentrations on microbial kinetics. Biotechnol Bioeng 12:679–712. https://doi.org/10.1002/bit.260120504
Duc HD (2023) Enhanced degradation of sulfamethoxazole using immobilized biomass reactor. Environ Eng Res 28(4):220213. https://doi.org/10.4491/eer.2022.213
Spain JC, Nishino SF (1987) Degradation of 1,4-dichlorobenzene by a Pseudomonas sp. Appl Environ Microbiol 53:1010–1019. https://doi.org/10.1128/aem.53.5.1010-1019.1987
Duc HD (2022) Anaerobic degradation of thiobencarb by mixed culture of isolated bacteria. FEMS Microbiol Lett. https://doi.org/10.1093/femsle/fnac123
Oanh NT, Duc HD (2022) Enhanced anaerobic degradation of thiobencarb using a horizontal-flow anaerobic immobilized biomass bioreactor. FEMS Microbiol Lett. https://doi.org/10.1093/femsle/fnac001
Ishikawa K, Nakamura Y, Kuwatsuka S (1976) Degradation of benthiocarb herbicide in soil. J Pestic Sci 1:49–57. https://doi.org/10.1584/jpestics.1.49
Haller WT, Stocker RK (2003) Toxicity of 19 adjuvants to juvenile Lepomis macrochirus (bluegill sunfish). Environ Toxicol Chem 22:615–619. https://doi.org/10.1002/etc.5620220321
Pérez-Bárcena JF, Ahuatzi-Chacón D, Castillo-Martínez KL, Ruiz-Ordaz N, Galíndez-Mayer J, Juárez-Ramírez C, Ramos-Monroy O (2014) Effect of herbicide adjuvants on the biodegradation rate of the methylthiotriazine herbicide prometryn. Biodegradation 25:405–415. https://doi.org/10.1007/s10532-013-9669-7
Duc HD, Thuy NTD, Truc HTT, Nhu NTH, Oanh NT (2020) Degradation of butachlor and propanil by Pseudomonas sp. strain But2 and Acinetobacter baumannii strain DT. FEMS Microbiol Lett 367(18):151. https://doi.org/10.1093/femsle/fnaa151
Andr J, Kočárek M, Jursík M, Fendrychová V, Tichý L (2017) Effect of adjuvants on the dissipation, efficacy and selectivity of three different pre-emergent sunflower herbicides. Plant Soil Environ 63:409–415. https://doi.org/10.17221/365/2017-PSE
Kucharski M, Sadowski J (2011) Behaviour of metazachlor applied with additives in soil–laboratory and field studies. J Food Agr Environ 9:723–726. https://doi.org/10.1016/J.Scitotenv.2007.01.021
Swarcewicz MK, Gregorczyk A (2013) Atrazine degradation in soil:effects of adjuvants and a comparison of three mathematical models. Pest Manag Sci 69:1346–1350. https://doi.org/10.1002/ps.3510
Duah-Yentumi S, Kuwatsuka S (1980) Effect of organic matter and chemical fertilizers on the degradation of benthiocarb and MCPA herbicides in the soil. Soil Sci Plant Nutr 26:541–549. https://doi.org/10.1080/00380768.1980.10431241
Duc HD (2022) Enhancement of carbofuran degradation by immobilized Bacillus sp. strain DT1. Environ Eng Res 27(4):210158. https://doi.org/10.4491/eer.2021.158
Acknowledgements
We are also grateful to the anonymous reviewers and the Executive Editor of Current Microbiology, whose suggestions helped to improve this manuscript.
Funding
This study was supported by the Vietnamese Ministry of Education and Training for the scientific theme (Code: B2022.SPD.04). The authors would like to thank them for all supports.
Author information
Authors and Affiliations
Contributions
HDD: designed the work plan and performed the experiments, prepared figures and tables. NTO and HDD: wrote this manuscript, reviewed and approved the final draft and approved the final version. NTMK: collected soil samples and prepared liquid media. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical Approval
The manuscript does not have potential conflicts of interest.
Research Involving Human and Animal Rights
The research does not involve human participants or animals.
Consent for Publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Duc, H.D., Oanh, N.T. & Khanh, N.T.M. Thiobencarb Degradation by Pseudomonas sp. Th1 and Cupriavidus oxalaticus Th2 Isolated from Soil. Curr Microbiol 80, 342 (2023). https://doi.org/10.1007/s00284-023-03456-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00284-023-03456-0