Abstract
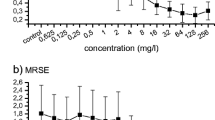
Staphylococcus aureus and methicillin-resistant S. aureus (MRSA) are known to be responsible of various infections, including biofilm-associated diseases. The aim of this study was to analyze 19 strains of S. aureus from orthopedic sites in terms of phenotypic antimicrobial susceptibility against 13 selected antibiotics, slime/biofilm formation, molecular analysis of specific antibiotic resistance genes (mecA, cfr, rpoB), and biofilm-associated genes (icaADBC operon). Furthermore, the effect of phloretin on the production of biofilm was evaluated on 8 chosen isolates. The susceptibility test confirmed almost all strains were resistant to cefoxitin and oxacillin. Most strains possess the mecA, whereas none of the strains had the cfr gene. Four strains (1, 7, 10, and 24) presented single-nucleotide polymorphisms (SNPs) in rpoB, which confer rifampicin resistance. IcaD was detected in all tested strains, whereas icaR was only found in two strains (24 and 30). Phloretin had a dose-dependent effect on biofilm production. Specifically, 0.5 × MIC determined biofilm inhibition in 5 out of 8 strains (8, 24, 25, 27, 30), whereas an increase in biofilm production was detected with phloretin at the 0.125 × MIC across all tested strains. These data are useful to potentially develop novel compounds against antibiotic-resistant S. aureus.


Similar content being viewed by others
Data Availability
Data available on request from the authors.
References
Tong SYC, Davis JS, Eichenberger E, Holland TL, Fowler VG (2015) Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev 3:603–661
Donlan RM, Costerton JW (2002) Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol 15:167–193
Keren I, Kaldalu N, Spoering A, Wang Y, Lewis K (2004) Persister cells and tolerance to antimicrobials. FEMS Microbiol Lett 230:13–18
Lewis K (2001) Riddle of biofilm resistance. Antimicrob Agents Chemother 45:999–1007
Xu Z, Xie L, Soteyome T, Peters BM, Shirtliff ME, LiuJ HJM (2019) Polymicrobial interaction and biofilms between Staphylococcus aureus and Pseudomonas aeruginosa: an underestimated concern in food safety. Curr Opin Food Sci 26:57–64
Arciola CR, Campoccia D, Ravaioli S, Montanaro L (2015) Polysaccharide intercellular adhesin in biofilm: structural and regulatory aspects. Front Cell Infect Microbiol 5:7
You Y, Xue T, Cao L, Zao L, Sun H, Sun B (2014) Staphylococcus aureus glucose-induced biofilm accessory proteins, GbaAB, influence biofilm formation in a PIA-dependent manner. Int J Mol Microbiol 304:603–612
Sionov RV, Steinberg D (2022) Targeting the holy triangle of quorum sensing, biofilm formation and antibiotic resistance in pathogenic bacteria. Microorganisms 10(6):1239
Lopes LAA, Dos Santos Rodrigues JB, Magnani M, de Souza EL, de Siqueira-Júnior JP (2017) Inhibitory effects of flavonoids on biofilm formation by Staphylococcus aureus that overexpresses efflux protein genes. Microb Pathog 107:193–197
Gao L, Tang Z, Li T, Wang J (2021) Combination of kaempferol and azithromycin attenuates Staphylococcus aureus-induced osteomyelitis via anti-biofilm effects and by inhibiting the phosphorylation of ERK1/2 and SAPK. Pathog Dis 79:ftab048
Barreca D, Bellocco E, Caristi C, Leuzzi U, Gattuso G (2011) Kumquat (Fortunella japonica Swingle) juice: flavonoid distribution and antioxidant properties. Food Res Int 44:2190–2197
Barreca D, Bellocco E, Laganà G, Ginestra G, Bisignano C (2014) Biochemical and antimicrobial activity of phloretin and its glycosilated derivatives present in apple and kumquat. Food Chem 160:292–297
Rezk BM, Haenen GRMM, Van der Vijgh WJF, Bast A (2002) The antioxidant activity of phloretin, the disclosure of a new antioxidant pharmacophore in flavonoids. Biochem Biophys Res Commun 295:9–13
Shuai-Cheng W, Ben-Dong F, Xiu-Ling C, Jian-Qing S, Yun-Xing F, Zhen-Qiang C, Dao-Xiu X, Zong-Mei W (2016) Subinhibitory concentrations of phloretin repressthe virulence of Salmonella typhimurium and protect against Salmonella typhimurium infection. Ant Van Leeuwenhoek 109(11):1503–1512
Adil M, Baig MH, Rupasinghe HPV (2019) Impact of citral and phloretin, alone and in combination, on major virulence traits of Streptococcus pyogenes. Molecules 24:4237
La Camera E, Bisignano C, Crisafi G, Smeriglio A, Denaro M, Trombetta D, Mandalari G (2018) Biochemical characterization of clinical strains of Staphylococcus spp. and their sensitivity to polyphenols-rich extracts from Pistachio (Pistacia vera L.). Pathogens 7:82
Bisignano C, Ginestra G, Smeriglio A, La Camera E, Crisafi G, Franchina FA, Tranchida PQ, Alibrandi A, Trombetta D, Mondello L, Mandalari G (2019) Study of the lipid profile of ATCC and clinical strains of Staphylococcus aureus in relation to their antibiotic resistance. Molecules 24:1276
EUCAST. The European committee on antimicrobial susceptibility Testing. Reference. Breakpoint tables for interpretation of MICs and zone diameters. Version 9.0. 1 January 2022. Available online: http://www.eucast.org. Accessed 24 Mar 2021
Nostro A, Guerrini A, Marino A, Tacchini M, Di Giulio M, Grandini A, Akin M, Cellini L, Bisignano G, Saraçoğlu HT (2016) In vitro activity of plant extracts against biofilm-producing food-related bacteria. Int J Food Microbiol 238:33–39
Clinical and Laboratory Standards Institute (CLSI) (2018) Performance Standards for Antimicrobial Susceptibility Testing. CLSI Approved Standard M100-S15. Clinical and Laboratory Standards Institute, Wayne.
Archer NK, Mazaitis MJ, Costerton JW, Leid JG, Powers ME, Shirtliff ME (2011) Staphylococcus aureus biofilms: properties, regulation, and roles in human disease. Virulence 2:445–459
Lane DJ (1991) 16S/23S rRNA sequencing. In: Stackebrandt E, Goodfellow M (eds) Nucleic Acids Techniques in Bacterial Systematics. Wiley, Chichester, pp 115–147
Osman K, Badr J, Al-Maary KS, Moussa IM, Hessain AM, Girah ZM, Abo-Shama UH, Orabi A, Saad A (2016) Prevalence of the antibiotic resistance genes in coagulase-positive-and negative-Staphylococcus in chicken meat retailed to consumers. Front Microbiol 7:1846
Aubry-Damon H, Soussy CJ, Courvalin P (1998) Characterization of mutations in the rpoB gene that confer rifampin resistance in Staphylococcus aureus. Antimicrob Agents Chemother 42:2590–2594
Jansen van Rensburg MJ, Whitelaw AC, Elisha BG (2012) Genetic basis of rifampicin resistance in methicillin-resistant Staphylococcus aureus suggests clonal expansion in hospitals in Cape Town. South Africa BMC Microb 12:46
Esteban J, Molina-Manso D, Spiliopoulou I, Cordero-Ampuero J, Fernández-Roblas R, Foka A, Gómez-Barrena E (2010) Biofilm development by clinical isolates of Staphylococcus spp. from retrieved orthopedic prostheses. Acta Orthop 81:674–679
Vestergaard M, Frees D, Ingmer H (2019) Antibiotic resistance and the MRSA problem. Microbiol Spec. https://doi.org/10.1128/microbiolspec.GPP3-0057-2018
Skov R, Larsen AR, Kearns A, Holmes M, Teale C, Edwards G, Hill R (2014) Phenotypic detection of mecC-MRSA: cefoxitin is more reliable than oxacillin. J Antimicrob Chemother 69:133–5
O’Neill AJ, Huovinen T, Fishwick CWG, Chopra I (2006) Molecular genetic and structural modeling studies of Staphylococcus aureus RNA polymerase and the fitness of rifampin resistance genotypes in relation to clinical prevalence. Antimicrob Agents Chemother 50(1):298–309
Guérillot R, Gonçalves da Silva A, Monk I, Giulieri S, Tomita T, Alison E, Porter J, Pidot S (2018) Convergent evolution driven by rifampin exacerbates the global burden of drug-resistant Staphylococcus aureus. mSphere 3(1):e00550-17
Wichelhaus T, Schäfer V, Brade V, Böddinghaus B (2001) Differential effect of rpoB mutations on antibacterial activities of rifampicin and KRM-1648 against Staphylococcus aureus. J Ant Chemot 47(2):153–156
Wang C, Fang R, Zhou B, Tian X, Zhang X, Zheng X, Zhang S, Dong G, Cao J, Zhou T (2019) Evolution of resistance mechanisms and biological characteristics of rifampicin-resistant Staphylococcus aureus strains selected in vitro. BMC Microbiol 19(1):220
Flemming HC, Wuertz S (2019) Bacteria andarchaea on Earth and their abundance in biofilms. Nature Rev Microbiol 17(4):247–260
Anderson GG, O’Toole GA (2008) Innate and induced resistance mechanisms of bacterial biofilms. Curr Topics Microbiol and Immunol 322:85–105
Hall CW, Mah TF (2017) Molecular mechanisms of biofilm-based antibiotic resistance and tolerance in pathogenic bacteria. FEMS Microbiol Rev 41(3):276–301
Wei LN, Shi CZ, Luo CX, Hu CY, Men YH (2020) Phloretin inhibits biofilm formation by affecting quorum sensing under different temperature. LWT-Food Sci Technol 131:109668
Acknowledgements
Not applicable.
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
“Conceptualization, GM, AN, OR, and DB; methodology, AM, ELC, and AN; writing—review and editing, GM, AN, OR, and DB; funding acquisition, GM and OR. All authors have read and agreed to the published version of the manuscript.”
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mandalari, G., Minuti, A., La Camera, E. et al. Antimicrobial Susceptibility of Staphylococcus aureus Strains and Effect of Phloretin on Biofilm Formation. Curr Microbiol 80, 303 (2023). https://doi.org/10.1007/s00284-023-03400-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00284-023-03400-2