Abstract
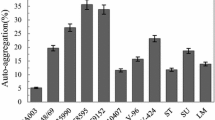
Enterococcus species are commensal organisms of the gastrointestinal tract and can also be isolated from traditional food products. They are used as probiotics in animals and less often in humans. This study aimed to investigate the antibacterial and anti-adhesive activities of twelve food-origin Enterococcus spp. biofilms on stainless steel AISI 316 L against foodborne pathogens, including Listeria monocytogenes CECT4032, Pseudomonas aeruginosa ATCC27853, and Escherichia coli ATCC25922. The antimicrobial and co-aggregation abilities of Enterococcus spp. were evaluated using spots-agar test and spectrophotometry aggregation assay, respectively. The anti-adhesive activity of selected strains on pathogenic bacteria was tested using serial dilution technique. Enterococci strains in planktonic mode showed strong inhibition activity against different pathogens tested with a significant difference in co-aggregation capacity. Moreover, L. monocytogenes and E. coli presented a low auto-aggregation rate compared to P. aeruginosa, which showed an amount of 11.25%. Scanning electron microscopy (SEM) revealed that biofilm biomass of Enterococcus spp. increased after 10 days. The thick layer of enterococci biofilms on AISI 316 L caused a low adhesion of L. monocytogenes, resulting in a reduction of approximately 2.8 log CFU/cm² for some selected strains. Additionally, Enterococcus monocultures’ biofilms were more efficient than polymicrobial cultures (a cocktail of enterococci strains) in controlling pathogen adhesion. These results indicate that monocultures of Enterococcus spp. biofilms could be used to prevent the adhesion of pathogenic bacteria on AISI 316 L.



Similar content being viewed by others
Data Availability
The datasets generated during the current study are available from the corresponding author on reasonable request.
References
Abee T, Kovács ÁT, Kuipers OP, Van der Veen S (2011) Biofilm formation and dispersal in Gram-positive bacteria. Curr Opin Biotechnol 22(2):172–179. https://doi.org/10.1016/j.lwt.2006.07.009
Dunne WM (2002) Bacterial adhesion: seen any good biofilms lately? Clin. Microbiol Rev 15(2):155–166. https://doi.org/10.1128/CMR.15.2.155-166.2002
Chen J, Rossman ML, Pawar DM (2007) Attachment of enterohemorrhagic Escherichia coli to the surface of beef and a culture medium. LWT 40(2):249–254. https://doi.org/10.1016/j.lwt.2005.10.011
Ouali FA, Al Kassaa I, Cudennec B, Abdallah M, Bendali F, Sadoun D, Chihib N-E, Drider D (2014) Identification of lactobacilli with inhibitory effect on biofilm formation by pathogenic bacteria on stainless steel surfaces. Int J Food Microbiol 191:116–124. https://doi.org/10.1016/j.ijfoodmicro.2014.09.011
Speranza B, Sinigaglia M, Corbo MR (2009) Non starter lactic acid bacteria biofilms: a means to control the growth of Listeria monocytogenes in soft cheese. Food Control 20:1063–1067. https://doi.org/10.1016/j.foodcont.2009.01.006
Martín-Espada M, D’ors A, Bartolomé M, Pereira M, Sánchez-Fortún S (2014) Peracetic acid disinfectant efficacy against Pseudomonas aeruginosa biofilms on polystyrene surfaces and comparison between methods to measure it. LWT 56:58–61. https://doi.org/10.1016/j.lwt.2013.11.013
Bore E, Langsrud S (2005) Characterization of micro-organisms isolated from dairy industry after cleaning and fogging disinfection with alkyl amine and peracetic acid. J Appl Microbiol 98:96–105. https://doi.org/10.1111/j.1365-2672.2004.02436.x
Al-Jailawi MH, Ameen RS, Al-Jeboori MR (2013) Effect of disinfectants on antibiotics susceptibility of Pseudomonas aeruginosa. J Appl Biotechnol 1(1):54–63. https://doi.org/10.5296/jab.v1i1.4038
Meyer B (2003) Approaches to prevention, removal and killing of biofilms. Int Biodeterior Biodegradation 51:249–253. https://doi.org/10.1016/S0964-8305(03)00047-7
Cacciatore FA, Brandelli A, Malheiros PdS (2021) Combining natural antimicrobials and nanotechnology for disinfecting food surfaces and control microbial biofilm formation. Crit Rev Food Sci Nutr 61:3771–3782. https://doi.org/10.1080/10408398.2020.1806782
Rahmati F, Hosseini SS, Safai SM, Lajayer BA, Hatami M (2020) New insights into the role of nanotechnology in microbial food safety. 3 Biotech 10(10):425. https://doi.org/10.1007/s13205-020-02409-9
Niaz B, Saeed F, Ahmed A, Imran M, Maan AA, Khan MKI, Tufail T, Anjum FM, Hussain S, Suleria HAR (2019) Lactoferrin (LF): a natural antimicrobial protein. Int J Food Prop 22(1):1626–1641. https://doi.org/10.1080/10942912.2019.1666137
Quintieri L, Caputo L, Monaci L, Cavalluzzi MM, Denora N (2020) Lactoferrin-derived peptides as a control strategy against Skinborne Staphylococcal Biofilms. Biomedicines 8(9). https://doi.org/10.3390/biomedicines8090323
Shahidi F, Roshanak S, Javadmanesh A, Tabatabaei Yazdi F, Pirkhezranian Z, Azghandi M (2020) Evaluation of antimicrobial properties of bovine lactoferrin against foodborne pathogenic microorganisms in planktonic and biofilm forms (in vitro). J Consum Prot Food Saf 15(3):277–283. https://doi.org/10.1007/s00003-020-01280-3
Hamadi F, Asserne F, Elabed S, Bensouda S, Mabrouki M, Latrache H (2014) Adhesion of Staphylococcus aureus on stainless steel treated with three types of milk. Food Control 38:104–108. https://doi.org/10.1016/j.foodcont.2013.10.006
Dat NM, Hamanaka D, Van Hung D, Tanaka F, Uchino T (2014) Surface conditioning of stainless steel coupons with skim milk, buttermilk, and butter serum solutions and its effect on bacterial adherence. Food Control 42:94–100. https://doi.org/10.1016/j.foodcont.2014.01.040
Hossain MI, Mizan MFR, Ashrafudoulla M, Nahar S, Joo H-J, Jahid IK, Park SH, Kim K-S, Ha S-D (2020) Inhibitory effects of probiotic potential lactic acid bacteria isolated from kimchi against Listeria monocytogenes biofilm on lettuce, stainless-steel surfaces, and MBEC™ biofilm device. LWT 118:108864. https://doi.org/10.1016/j.lwt.2019.108864
Speranza B, Liso A, Russo V, Corbo MR (2020) Evaluation of the potential of Biofilm formation of Bifidobacterium longum subsp. infantis and Lactobacillus reuteri as competitive Biocontrol Agents against pathogenic and food spoilage Bacteria. Microorganisms 8(2). https://doi.org/10.3390/microorganisms8020177
Gómez NC, Ramiro JMP, Quecan BXV, de Melo Franco BDG (2016) Use of potential probiotic lactic acid Bacteria (LAB) Biofilms for the control of Listeria monocytogenes, Salmonella Typhimurium, and Escherichia coli O157:H7 biofilms formation. Front Microbiol 7:863. https://doi.org/10.3389/fmicb.2016.00863
Nebbia S, Lamberti C, Lo Bianco G, Cirrincione S, Laroute V, Cocaign-Bousquet M, Cavallarin L, Giuffrida MG, Pessione E (2021) Antimicrobial potential of food lactic acid Bacteria: bioactive peptide decrypting from Caseins and Bacteriocin Production. Microorganisms 9(1). https://doi.org/10.3390/microorganisms9010065
Melo TA, Dos Santos TF, de Almeida ME, Junior LA, Andrade EF, Rezende RP, Marques LM, Romano CC (2016) Inhibition of Staphylococcus aureus biofilm by Lactobacillus isolated from fine cocoa. BMC Microbiol 16(1):250. https://doi.org/10.1186/s12866-016-0871-8
Walencka E, Rozalska S, Sadowska B, Rozalska B (2008) The influence of Lactobacillus acidophilus-derived surfactants on staphylococcal adhesion and biofilm formation. Folia Microbiol 53(1):61–66. https://doi.org/10.1007/s12223-008-0009-y
Giraffa G (2003) Functionality of enterococci in dairy products. Int J Food Microbiol 88:215–222. https://doi.org/10.1016/S0168-1605(03)00183-1
Ait Meddour A, Bendali F, Sadoun D (2015) Anti-adherence potential of Enterococcus durans cells and its cell-free supernatant on plastic and stainless steel against foodborne pathogens. Folia Microbiol 60:357–363. https://doi.org/10.1007/s12223-014-0367-6
Achemchem F, Abrini J, Martinez-Bueno M, Valdivia E, Maqueda M (2006) Control of Listeria monocytogenes in goat’s milk and goat’s Jben by the bacteriocinogenic Enterococcus faecium F58 strain. J Food Prot 69:2370–2376. https://doi.org/10.4315/0362-028X-69.10.2370
Achemchem F, Martínez-Bueno M, Abrini J, Valdivia E, Maqueda M (2005) Enterococcus faecium F58, a bacteriocinogenic strain naturally occurring in Jben, a soft, farmhouse goat’s cheese made in Morocco. J Appl Microbiol 99:141–150. https://doi.org/10.1111/j.1365-2672.2005.02586.x
Elmoslih A, Zanzan M, Aissa R, Hamadi F, Ait Baddi G, Ait Ben Aoumar A, Achemchem F (2017) Isolation and characterization of bacteriocinogenic enterococcal and lactococcal strains from south of Morocco dairy product. J Int 18:2456–7051. https://doi.org/10.9734/bji/2017/32919
Achemchem F, Cebrián R, Abrini J, Martínez-Bueno M, Valdivia E, Maqueda M (2012) Antimicrobial characterization and safety aspects of the bacteriocinogenic Enterococcus hirae F420 isolated from moroccan raw goat milk. Can J Microbiol 58:596–604. https://doi.org/10.1139/w2012-027
Collado MC, Meriluoto J, Salminen S (2008) Adhesion and aggregation properties of probiotic and pathogen strains. Eur Food Sci Technol 226:1065–1073. https://doi.org/10.1007/s00217-007-0632-x
Tuo Y, Yu H, Ai L, Wu Z, Guo B, Chen W (2013) Aggregation and adhesion properties of 22 Lactobacillus strains. Int J Dairy Sci 96:4252–4257. https://doi.org/10.3168/jds.2013-6547
Akbas MY, Cag S (2016) Use of organic acids for prevention and removal of Bacillus subtilis biofilms on food contact surfaces. Food Sci Technol Int 22:587–597. https://doi.org/10.1177/1082013216633545
Jia R, Yang D, Li Y, Xu D, Gu T (2017) Mitigation of the Desulfovibrio vulgaris biofilm using alkyldimethylbenzylammonium chloride enhanced by D-amino acids. Int Biodeterior Biodegradation 117:97–104. https://doi.org/10.1016/j.ibiod.2016.12.001
Teixeira P, Silva SC, Araújo F, Azeredo J, Oliveira R (2007) Bacterial adhesion to food contacting surfaces. In: Méndez-Vilas A (ed) Communicating Current Research and Educational Topics and Trends in Applied Microbiology. Formatex, Badajoz, pp 13–20. http://hdl.handle.net/1822/7711
Lee B-H, Hébraud M, Bernardi T (2017) Increased adhesion of Listeria monocytogenes strains to abiotic surfaces under cold stress. Front Microbiol 8:2221. https://doi.org/10.3389/fmicb.2017.02221
Silva S, Teixeira P, Oliveira R, Azeredo J (2008) Adhesion to and viability of Listeria monocytogenes on food contact surfaces. J Food Prot 71(7):1379–1385. https://doi.org/10.4315/0362-028X-71.7.1379
Aguilar C, Vanegas C, Klotz B (2011) Antagonistic effect of Lactobacillus strains against Escherichia coli and Listeria monocytogenes in milk. J Dairy Res 78:136–143. https://doi.org/10.1017/S0022029910000877
Semedo T, Santos MA, Lopes MF, Marques JJF, Crespo MT, Tenreiro R (2003) Virulence factors in food, clinical and reference enterococci: a common trait in the genus? Syst Appl Microbiol 26:13–22. https://doi.org/10.1078/072320203322337263
Onda T, Yanagida F, Uchimura T, Tsuji M, Ogino S, Shinohara T, Yokotsuka K (2002) Widespread distribution of the bacteriocin-producing lactic acid cocci in Miso‐paste products. J Appl Microbiol 92:695–705. https://doi.org/10.1046/j.1365-2672.2002.01573.x
Guerrieri E, de Niederhäusern S, Messi P, Sabia C, Iseppi R, Anacarso I, Bondi M (2009) Use of lactic acid bacteria (LAB) biofilms for the control of Listeria monocytogenes in a small-scale model. Food Control 20(9):861–865. https://doi.org/10.1016/j.foodcont.2008.11.001
Shokri D, Khorasgani MR, Mohkam M, Fatemi SM, Ghasemi Y, Taheri-Kafrani A (2018) The inhibition effect of Lactobacilli against Growth and Biofilm formation of Pseudomonas aeruginosa. Probiotics Antimicrob Proteins 10:34–42. https://doi.org/10.1007/s12602-017-9267-9
Jabalameli F, Mirsalehian A, Khoramian B, Aligholi M, Khoramrooz SS, Asadollahi P, Taherikalani M, Emaneini M (2012) Evaluation of biofilm production and characterization of genes encoding type III secretion system among Pseudomonas aeruginosa isolated from burn patients. Burns 38:1192–1197. https://doi.org/10.1016/j.burns.2012.07.030
Corehtash ZG, Khorshidi A, Firoozeh F, Akbari H, Aznaveh AM (2015) Biofilm formation and virulence factors among Pseudomonas aeruginosa isolated from burn patients. Jundishapur J Microbiol 8(10):e22345. https://doi.org/10.5812/jjm.22345
Jacobs A, Chenia H (2009) Biofilm-forming capacity, surface hydrophobicity and aggregation characteristics of Myroides odoratus isolated from south african Oreochromis mossambicus fish. J Appl Microbiol 107:1957–1966. https://doi.org/10.1111/j.1365-2672.2009.04380.x
Sorroche FG, Spesia MB, Zorreguieta Á, Giordano W (2012) A positive correlation between bacterial autoaggregation and biofilm formation in native Sinorhizobium meliloti isolates from Argentina. Appl Environ Microbiol 78:4092–4101. https://doi.org/10.1128/AEM.07826-11
Trunk T, Salah Khalil H, Leo JC (2018) Bacterial autoaggregation. AIMS Microbiol 4:140–164. https://doi.org/10.3934/microbiol.2018.1.140
Kragh KN, Hutchison JB, Melaugh G, Rodesney C, Roberts AE, Irie Y, Jensen P, Diggle SP, Allen RJ, Gordon V (2016) Role of multicellular aggregates in biofilm formation. MBio 7:e00237–e00216. https://doi.org/10.1128/mBio.00237-16
Ndahetuye JB, Koo OK, O’Bryan CA, Ricke SC, Crandall PG (2012) Role of lactic acid bacteria as a biosanitizer to prevent attachment of Listeria monocytogenes F6900 on deli slicer contact surfaces. J Food Prot 75:1429–1436. https://doi.org/10.4315/0362-028X.JFP-12-072
Jin L, Marquardt R, Zhao X (2000) A strain of Enterococcus faecium (18C23) inhibits adhesion of enterotoxigenic Escherichia coli K88 to porcine small intestine mucus. Appl Environ Microbiol 66:4200–4204. https://doi.org/10.1128/AEM.66.10.4200-4204.2000
Acknowledgements
The authors would like to thank Prof. Naima Trimasse from Ibn Zohr University for her valuable English language corrections.
Funding
Mariem Zanzan was the beneficiary of a research scholarship from the National Center for Scientific and Technical Research (Number: 18UIZ2016, CNRST, Morocco).
Author information
Authors and Affiliations
Contributions
This work is the result of collaboration between all the authors: MZ: Methodology, investigation and writing the first draft of the manuscript. FA and FH: Conceptualization, methodology, validation, resources and writing- reviewing & editing the final manuscript. HL: Methodology and validation of the pathogenic biofilms experiments. AE: Methodology and investigation on lactic acid bacteria used in this work. RM: Reviewing and editing the final manuscript and supervision. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zanzan, M., Achemchem, F., Hamadi, F. et al. Anti-adherence Activity of Monomicrobial and Polymicrobial Food-Derived Enterococcus spp. Biofilms Against Pathogenic Bacteria. Curr Microbiol 80, 216 (2023). https://doi.org/10.1007/s00284-023-03326-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00284-023-03326-9