Abstract
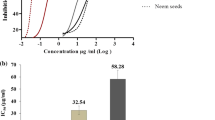
Drug resistance to practically all antimalarial drugs in use necessitate the development of new chemotherapeutics against malaria. In this aspect, traditionally used plants with folklore reputation are the pillar for drug discovery. Cuscuta reflexa being traditionally used in the treatment of malaria in Odisha, India we aimed to experimentally validate its antimalarial potential. Different solvent extracts of C. reflexa or column fractions from a promising solvent extract were evaluated for in vitro anti-plasmodial activity against Plasmodium falciparum strain Pf3D7. Potent fractions were further evaluated for inhibition of parasite growth against different drug resistant strains. Safety of these fractions was determined by in vitro cyto-toxicity, and therapeutic effectiveness was evaluated by suppression of parasitemia and improvement in survival of experimental mice. Besides, their immunomodulatory effect was investigated in Pf-antigen stimulated RAW cells. GCMS fingerprints of active fractions was determined. Column separation of methanol extract which showed the highest in vitro antiplasmodial activity (IC50 = 14.48 μg/ml) resulted in eleven fractions, three of which (F2, F3, and F4) had anti-plasmodial IC50 ranging from ≤ 10 to 2.2 μg/ml against various P. falciparum strains with no demonstration of in vitro cytotoxicity. F4 displayed the highest in vivo parasite suppression, and had a mean survival time similar to artesunate (19.3 vs. 20.6 days). These fractions significantly modulated expression of inflammatory cytokines in Pf-antigen stimulated RAW cells. The findings of the study confirm the antimalarial potential of C. reflexa. Exploration of phyto-molecules in GCMS fingerprints of active fractions is warranted for possible identification of lead anti-malarial phyto-drugs.


Similar content being viewed by others
Data Availability
All data are available with corresponding authors.
Code Availability
Not applicable.
References
Dondorp AM, Fanello CI, Hendriksen IC, Gomes E et al (2010) Artesunate versus quinine in the treatment of severe falciparum malaria in African children (AQUAMAT): an open-label, randomised trial. Lancet 376(9753):1647–1657. https://doi.org/10.1016/s0140-6736(10)61924-1
Oluwayemi IO, Brown BJ, Oyedeji OA, Oluwayemi MA (2013) Neurological sequelae in survivors of cerebral malaria. Pan Afr Med J. https://doi.org/10.11604/pamj.2013.15.88.1897
Schiess N, Villabona-Rueda A, Cottier KE, Huether K et al (2020) Pathophysiology and neurologic sequelae of cerebral malaria. Malar J 19(1):1–12. https://doi.org/10.1186/s12936-020-03336-z
Chua CLL, Ng IMJ, Yap BJM, Teo A (2021) Factors influencing phagocytosis of malaria parasites: the story so far. Malar J 20(1):1–15. https://doi.org/10.1186/s12936-021-03849-1
Dobbs KR, Crabtree JN, Dent AE (2020) Innate immunity to malaria—the role of monocytes. Immunol Rev 293(1):8–24. https://doi.org/10.1111/imr.12830
Gbedande K, Carpio VH, Stephens R (2020) Using two phases of the CD 4 T cell response to blood-stage murine malaria to understand regulation of systemic immunity and placental pathology in Plasmodium falciparum infection. Immunol Rev 293(1):88–114. https://doi.org/10.1111/imr.12835
Leão L, Puty B, Dolabela MF, Povoa MM et al (2020) Association of cerebral malaria and TNF-α levels: a systematic review. BMC Infect Dis 20(1):1–17. https://doi.org/10.1186/s12879-020-05107-2
Popa GL, Popa MI (2021) Recent advances in understanding the inflammatory response in malaria: a review of the dual role of cytokines. J Immunol Res. https://doi.org/10.1155/2021/7785180
Woodrow CJ, White NJ (2017) The clinical impact of artemisinin resistance in Southeast Asia and the potential for future spread. FEMS Microbiol Rev 41(1):34–48. https://doi.org/10.1093/femsre/fuw037
Ikeda M, Kaneko M, Tachibana SI, Balikagala B et al (2018) Artemisinin-resistant Plasmodium falciparum with high survival rates, Uganda, 2014–2016. Emerg Infect Dis 24(4):718. https://doi.org/10.3201/eid2404.170141
El-Sheikha AF (2017) Medicinal plants: ethno-uses to biotechnology era. Biotechnology and Production of Anti-Cancer Compounds. https://doi.org/10.1007/978-3-319-53880-8_1
Al-Sokari S, El-Sheikha FA (2015) In vitro antimicrobial activity of crude extracts of some medicinal plants from Al-Baha region in Saudi Arabia. J Food Nutr Sci 3(1):74. https://doi.org/10.11648/j.jfns.s.2015030102.24
Woon-Chien T et al (2016) Research on medicinal plants for malaria. Medicinal Plants Malaria. https://doi.org/10.1201/b19026-3
Karunamoorthi K, Sabesan S, Jegajeevanram K, Vijayalakshmi J (2013) Role of traditional antimalarial plants in the battle against the global malaria burden. Vector-Borne Zoonotic Dis 13(8):521–544. https://doi.org/10.1089/vbz.2011.0946
Rout S, Panda S (2010) Ethno-medicinal plant resources of Mayurbhanj district, Orissa. https://doi.org/10.1080/09735070.2009.11886333
Bhan S, Mohan L, Srivastava CN (2015) Efficacy of Cuscuta reflexa extract and its synergistic activity with Temephos against mosquito larvae. Int J Mosq Res 2:34–41
Mishra S, Alhodieb FS, Barkat MA, Hassan MZ et al (2022) Antitumor and hepatoprotective effect of Cuscuta reflexa Roxb in a murine model of colon cancer. J Ethnopharmacol 282:114597. https://doi.org/10.1016/j.jep.2021.114597
Verma N, Yadav RK (2018) Cuscuta reflexa: a paracitic medicinal plant. Plant Arch 18:1938–1942
Ojha SB, Roy S, Das S, Dhangadamajhi G (2019) Phytochemicals screening, phenolic estimation and evaluation for anti-oxidant, anti-inflammatory and anti-microbial activities of sequentially Soxhlet extracted coconut testa. Food Nutr Sci 10(08):900. https://doi.org/10.4236/fns.2019.108065
Ginovyan M, Ayvazyan A, Nikoyan A, Tumanyan L et al (2020) Phytochemical screening and detection of antibacterial components from crude extracts of some armenian herbs using TLC-bioautographic technique. Curr Microbiol 77(7):1223–1232. https://doi.org/10.1007/s00284-020-01929-0
Komsta L, Waksmundzka-Hajnos M, Sherma J (eds) (2013). CRC Press, p 20
Zhu Y-P, Song Y-R, Quan W, Xu X-X et al (2021) Letter to the editor: administration of TGF-ß inhibitor mitigates radiation-induced fibrosis in a mouse model. Clin Orthop Relat Res 479(8):1862–1863. https://doi.org/10.1097/corr.0000000000001815
Sah RK, Pati S, Saini M, Singh S (2021) Erythrocyte sphingosine kinase regulates intraerythrocytic development of Plasmodium falciparum. Sci Rep 11(1):1–16. https://doi.org/10.1038/s41598-020-80658-7
Ramu D, Jain R, Kumar RR, Sharma V et al (2019) Design and synthesis of imidazolidinone derivatives as potent anti-leishmanial agents by bioisosterism. Arch Pharm (Weinheim) 352(4):1800290. https://doi.org/10.1002/ardp.201800290
Chaurasiya A, Garg S, Khanna A, Narayana C et al (2021) Pathogen induced subversion of NAD+ metabolism mediating host cell death: a target for development of chemotherapeutics. Cell Death Discov 7(1):1–21. https://doi.org/10.1038/s41420-020-00366-z
Madan E, Puri M, Muthuswami R, Zilberstein D et al (2021) Leishmania parasite arginine deprivation response pathway influences the host macrophage lysosomal arginine sensing machinery. BioRxiv. https://doi.org/10.1101/2021.09.01.458453
Fidock DA, Rosenthal PJ, Croft SL, Brun R et al (2004) Antimalarial drug discovery: efficacy models for compound screening. Nat Rev Drug Discov 3(6):509–520. https://doi.org/10.1038/nrd1416
Peters W, Fleck S, Robinson B, Stewart L et al (2002) The chemotherapy of rodent malaria LX: The importance of formulation in evaluating the blood schizontocidal activity of some endoperoxide antimalarials. Ann Trop Med Parasitol 96(6):559–573. https://doi.org/10.1179/000349802125001744
Moros G, Chatziioannou AC, Gika HG, Raikos N et al (2017) Investigation of the derivatization conditions for GC–MS metabolomics of biological samples. Bioanalysis 9(1):53–65. https://doi.org/10.4155/bio-2016-0224
Gokhale M, Gautam D, Khanna A (2017) A comparative GC-MS analysis of bioactive compounds in the different fractions of root extract of oroxylum indicum (L) vent. Anal Chem Lett 7(3):410–20. https://doi.org/10.1080/22297928.2017.1351889
Lee DK, In J, Lee S (2015) Standard deviation and standard error of the mean. Korean J Anesthesiol 68(3):220. https://doi.org/10.4097/kjae.2015.68.3.220
Habibi P, Shi Y, Fatima Grossi-de-Sa M, Khan I (2022) Plants as sources of natural and recombinant antimalaria agents. Mol Biotechnol 64(11):1177–1197. https://doi.org/10.1016/j.biotechadv.2018.02.002
World Health Organisation (2015) Guidelines for the treatment of malaria. World Health Organization. https://apps.who.int/iris/handle/10665/162441
Sowunmi A, Walker O, Salako L (1992) Hyperparasitaemia: not a reliable indicator of severity or poor prognosis in falciparum malaria in children in endemic African countries. Ann Trop Paediatr 12(2):155–158. https://doi.org/10.1080/02724936.1992.11747561
Malaguarnera L, Pignatelli S, Musumeci M, Simporè J et al (2002) Plasma levels of interleukin-18 and interleukin-12 in Plasmodium falciparum malaria. Parasite Immunol 24(9–10):489–492. https://doi.org/10.1046/j.1365-3024.2002.00485.x
Dodoo D, Omer F, Todd J, Akanmori B et al (2002) Absolute levels and ratios of proinflammatory and anti-inflammatory cytokine production in vitro predict clinical immunity to Plasmodium falciparum malaria. J Infect Dis 185(7):971–979. https://doi.org/10.1086/339408
Mitchell AJ, Hansen AM, Hee L, Ball HJ et al (2005) Early cytokine production is associated with protection from murine cerebral malaria. Infect Immun 73(9):5645–5653. https://doi.org/10.1128/iai.73.9.5645-5653.2005
Chatterjee D, Sahu RK, Jha AK, Dwivedi J (2011) Evaluation of antitumor activity of Cuscuta reflexa Roxb (Cuscutaceae) against Ehrlich ascites carcinoma in Swiss albino mice. Trop J Pharm Res 10(4):447–454. https://doi.org/10.4314/tjpr.v10i4.10
Thomas S, Shrikumar S, Velmurugan C, Kumar BA (2015) Evaluation of anxiolytic effect of whole plant of Cuscuta reflexa. World J Pharm Sci 4:1245–1253
Amlabu WE, Nock IH (2018) Antimalarial efficacy of Vitellaria paradoxa Gaertn (Family: Sapotaceae) leaves and stem bark. FUW Trends Sci Technol J 3:605–609
Ezim O, Alagbe O, Idih F (2021) Antimalarial activity of ethanol extract of Mucuna pruriens leaves on Nk65 Chloroquine sensitive strain of plasmodium berghei. J Complement Altern Med Res 13(4):1–7. https://doi.org/10.9734/jocamr/2021/v13i430229
Fujisaki R, Kamei K, Yamamura M, Nishiya H et al (2012) In vitro and in vivo anti-plasmodial activity of essential oils, including hinokitiol. Southeast Asian J Trop Med Public Health 43(2):270–279
Khan H, Saeed M, Muhammad N, Tariq SA et al (2013) Antimalarial and free radical scavenging activities of aerial parts of Polygonatum verticillatum (L.) All. and identification of chemical constituents by GC-MS. Pak J Bot 45:497–500. https://doi.org/10.1007/s00044-011-9637-x
Khan M (2016) Antimalarial, non alkaloidal molecules from preliminary elucidation of nauclea diderechi extract. J Pharm Pharma 3:1–7. https://doi.org/10.15436/2377-1313.16.012
Lam NS, Long X, Su X, zhuan, Lu F, (2020) Melaleuca alternifolia (tea tree) oil and its monoterpene constituents in treating protozoan and helminthic infections. Biomed Pharmacother 130:110624. https://doi.org/10.1016/j.biopha.2020.110624
Wangchuk P, Keller PA, Pyne SG, Taweechotipatr M et al (2013) GC/GC-MS analysis, isolation and identification of bioactive essential oil components from the Bhutanese medicinal plant Pleurospermum amabile. Nat Prod Commun 8(9):1934578X1300800930. https://doi.org/10.1177/1934578x1300800930
Sachdeva C, Mohanakrishnan D, Kumar S, Kaushik NK (2020) Assessment of in vitro and in vivo antimalarial efficacy and GC-fingerprints of selected medicinal plant extracts. Exp Parasitol 219:108011. https://doi.org/10.1016/j.exppara.2020.108011
Prakash V (2022) To perform gas chromatography and Mass spectroscopy (GC-MS) analysis of Achyranthes aspera L leaf extract. J Drug Deliv Therap. 12(1-S):1–3. https://doi.org/10.22270/jddt.v12i1-s.5299
Rasoanaivo P, Wright CW, Willcox ML, Gilbert B (2011) Whole plant extracts versus single compounds for the treatment of malaria: synergy and positive interactions. Malaria J. https://doi.org/10.1186/1475-2875-10-s1-s4
Willcox M (2011) Improved traditional phytomedicines in current use for the clinical treatment of malaria. Planta Med 77(06):662–671. https://doi.org/10.1055/s-0030-1250548
Acknowledgements
We thankfully acknowledge the financial support from Science and Technology Department, Govt. of Odisha to GD and from Drug and Pharmaceuticals Research Programe (DPRP) to SS. SBO is supported by DST-INSPIRE fellowship. We sincerely thank Advanced Instrumentation and Research Facility (AIRF), JNU, New Delhi for providing the facility of GCMS/MS. We thank Central Instrumentation Facility (CIF) of Special Centre for Molecular Medicine (SCMM), JNU New Delhi.
Funding
This study is supported by the Science and Technology Department, Govt. of Odisha to GD (27552800232014/20) and from Drug and Pharmaceuticals Research Program to SS (P/569/2016–1/TDT, SS).
Author information
Authors and Affiliations
Contributions
Conceptualization, GD and SS; Methodology, SS, GD, SBO and RS; Investigation, SBO, RS, EM, RB and SR; Writing- original draft, SBO, GD and SS; Writing-review and editing, SS and GD. Funding acquisition, SS and GD; Supervision, SS and GD. All authors contributed to the article and approved the submitted version.
Corresponding authors
Ethics declarations
Conflict of interest
All authors have no conflict of interest.
Ethical Approval
Approved by IAEC of Jawaharlal Nehru University (JNU), Delhi (JNU/IBSC/2020/18).
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ojha, S.B., Sah, R.K., Madan, E. et al. Cuscuta reflexa Possess Potent Inhibitory Activity Against Human Malaria Parasite: An In Vitro and In Vivo Study. Curr Microbiol 80, 189 (2023). https://doi.org/10.1007/s00284-023-03289-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00284-023-03289-x