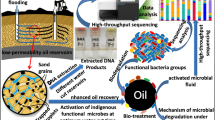
Abstract
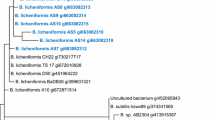
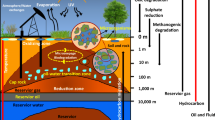
Crude oil is a primary energy source used for economic expansion across the world. Secondary recovery processes employed by industries to recover oil from oil wells leave behind 70% of the oil trapped in marginal and deleted zones of reservoirs. To recover the oil from depleted zones, microbial enhanced oil recovery (MEOR) tertiary processes were introduced, which involve the production of metabolites from the indigenous microbiome. In this study, the indigenous microbiota was identified as Marinobacterium sp., Silvanigrella sp., Petrothermobacter sp., Pseudomonas sp., Bacillus sp., Nitrincola sp., Halomonas sp., Uncultured Roseovarius sp., and Phaeobacter. Further, the secondary metabolites such as volatile fatty acids (ethanol, acetone, and acetate), biomass, gases (CO2, CH4), and biosurfactants were estimated through gas chromatography and FTIR spectroscopy. Once stable microbial growth was attained in the baltch media, it was optimized through response surface methodology (RSM) to minimize the process cost. The optimized media with 9 g/L of molasses, 1.75 g/L of sodium bicarbonate, and 1.25 g/L of ammonium chloride showed a significant impact on metabolite production. Additionally, core flood studies to simulate field studies were performed that represented that TeriK-1 brought a significant increment of 18.9%, which makes it suitable for MEOR field implementation. This study is one of its kind where the indigenous thermophilic sp. was successfully established and is capable of producing the secondary metabolites that aid in the MEOR process.
Graphical abstract






Similar content being viewed by others
References
Mcdonald CP, Lottig NR, Stoddard J, LComment on Bachmann, et al (2014) (2013): a non-representative sample cannot describe the extent of cultural eutrophication of natural lakes in the United States. Limnol Oceanogr 59:2226–2230. https://doi.org/10.4319/lo.2014.59.6.2226
Safdel M, Anbaz MA, Daryasafar A, Jamialahmadi MJ (2017) Microbial enhanced oil recovery, a critical review on worldwide implemented field trials in different countries. Renew Sust Energ Rev 74:159–172. https://doi.org/10.1016/j.rser.2017.02.045
Gieg LM, Jack TR, Foght JM (2011) Biological souring and mitigation in oil reservoirs. Appl Microbiol Biotechnol 92:263–282. https://doi.org/10.1007/s00253-011-3542-6
Brzeszcz J, Kaszycki P (2018) Aerobic bacteria degrading both n-alkane and aromatic hydrocarbons: an undervalued strategy for metabolic diversity and flexibility. Biodegradation 29:359–407. https://doi.org/10.1007/s10532-018-9837-x
Shibulal B, Bahry SN, Al-Wahaibi YM, Elshafie AE, Bemani AS, Al- Bemani AS (2014) Microbial enhanced heavy oil recovery by the aid of inhabitant spore-forming bacteria: an insight review. Sci World J 2014:309159. https://doi.org/10.1155/2014/309159
Gao CH, Zekri A (2011) Applications of microbial-enhanced oil recovery technology in the past decade. Energy Sour Part A Recov Util Environ Eff 33:972–989. https://doi.org/10.1080/15567030903330793
Niu J, Liu Q, Lv J, Peng B (2020) Review on microbial enhanced oil recovery: Mechanisms, modeling and field trials. J Pet Sci Eng 192:107350. https://doi.org/10.1016/j.petrol.2020.107350
Zhang XY, Liu XY, Zhong CL, Cao ZG, Liu FH, Chen LS, Liu SS, Yan HJ (2012) Soil microbial community response to pyrene at the presence of Scirpustriqueter. Eur J Soil Biol 50:44–50. https://doi.org/10.1016/j.ejsobi.2011.12.009
Li H, Yang S, Mu B, Rong Z, Zhang J (2007) Molecular phylogenetic diversity of the microbial community associated with a high-temperature petroleum reservoir at an offshore oilfield. FEMS Microbiol Ecol 60:74–84. https://doi.org/10.1111/j.1574-6941.2006.00266.x
Gao PK, Li GQ, Tian HM, Wang YS (2015) Differences in microbial community composition between injection and production water samples of water flooding petroleum reservoirs. Biogeosciences 12:3403–3414. https://doi.org/10.5194/bg-12-3403-2015
Zhou JF, Gao PK, Dai XH, Cui XY, Tian HM, Xie JJ, Li GQ, Ma T (2018) Heavy hydrocarbon degradation of crude oil by a novel thermophilic Geobacillusstearothermophilus strain A-2. Int Biodeterior Biodegrad 126:224–230. https://doi.org/10.1016/j.ibiod.2016.09.031
VanHamme JD, Singh A, Ward OP (2003) Recent advances in petroleum microbiology. Microbiol Mol Biol Rev 67:503–549. https://doi.org/10.1128/MMBR.67.4.503-549.2003
Singla RK, Dubey HD, Dubey AK (2014) Therapeutic spectrum of bacterial metabolites. Indo Global J Pharmaceut Sci 2:52–64
Wu YS, Ngai SC, Goh BH, Chan KG, Lee LH, Lay-Hong Chuah LH (2017) Anticancer activities of surfactin and potential application of nanotechnology assisted surfactin delivery. Front Pharmacol 8:761. https://doi.org/10.3389/fphar.2017.00761
Gudina EJ, Rangarajan V, Sen R, Rodrigues LR (2013) Potential therapeutic applications of biosurfactants. Trends Pharmacol Sci 34:667–675. https://doi.org/10.1016/j.tips.2013.10.002
Liu JF, Mbadinga SM, Yang SZ, Gu JD, Bo-Zhong MBZ (2015) Chemical structure, property and potential applications of biosurfactants produced by Bacillus subtilis in petroleum recovery and spill mitigation. Int J Mol Sci 16:4814–4837. https://doi.org/10.3390/ijms16034814
Abdel MAM, Aboulwafa MM, Hassouna NA (2008) Characterization of surfactin produced by Bacillus subtilis isolate BS5. Appl Biochem Biotechnol 150:289–303. https://doi.org/10.1007/s12010-008-8153-z
Sharma N, Lavania M, Lal B (2022) Biosurfactant: a next generation tool for sustainable remediation of organic pollutants. Front Microbiol 12:821531. https://doi.org/10.3389/fmicb.2021.821531
Banat IM, Franzetti A, Gandolfi I, Bestetti G, Martinotti MG, Fracchia L et al (2010) Microbial biosurfactants production, applications and future potential. Appl Microbiol Biotechnol 87:427–444. https://doi.org/10.1007/s00253-010-2589-0
Hosseininoosheri P, Lashgari HR, Sepehrnoori K (2016) A novel method to model and characterize in-situ bio-surfactant production in microbial enhanced oil recovery. Fuel 183:501–511. https://doi.org/10.1016/j.fuel.2016.06.035
Zhao F, Li P, Guo C, Shi RJ, Zhang Y (2017) Bioaugmentation of oil reservoir indigenous Pseudomonas aeruginosa to enhance oil recovery through in-situ biosurfactant production without air injection. Bioresour Technol 251:295–330. https://doi.org/10.1016/j.biortech.2017.12.057
McInerney MJ, Nagle DP, KnappR M (2005) Microbially enhanced oil recovery: past, present, and future. In: Ollivier B, Magot M (eds) Petroleum Microbiology. ASM Press, Washington, DC, pp 215–237
Youssef N, Simpson DR, Duncan KE, McInerney MJ, Folmsbee M, Fincher T, Knapp RM (2007) In situ biosurfactant production by Bacillus strains injected into a limestone petroleum reservoir. Appl Environ Microbial 73(4):1239–1247. https://doi.org/10.1128/AEM.02264-06
TianY XS, Ma Y (2020) Comparative analysis of bacterial community and functional sp. in oil reservoirs with different in situ temperatures. Int Microbiol 23:557–563. https://doi.org/10.1007/s10123-020-00125-1
Sharma N, Lavania M, Kukreti V, Rana DP (2018) Laboratory investigation of Indigenous Consortia TERIJ-188 for incremental oil recovery. Front Microbiol 8(9):2357. https://doi.org/10.3389/fmicb.2018.02357
Rathi R, Lavania M, Singh N, Sarma PM, Kishore P, Hajra P, Lal B (2019) Evaluating indigenous diversity and its potential for microbial methane generation from thermogenic coal bed methane reservoir. Fuel 250:362–372. https://doi.org/10.1016/j.fuel.2019.03.125
Lavania M, Cheema S, Lal B (2015) Potential of viscosity reducing thermophillic anaerobic bacterial consortium TERIB#90 in upgrading heavy oil. Fuel 144:349–357. https://doi.org/10.1016/J.FUEL.2014.12.003
Basera P, Lavania M, Lal B (2019) Potential of dynamic communities in the bio-corrosion process: a proof study with surface morphology of metal coupons. RSC Adv 9:17040–17050. https://doi.org/10.1039/C9RA01959F
Ke CY, Lu GM, Li YB, Sun WJ, Zhang QZ, Zhang XL (2018) A pilot study on large-scale microbial enhanced oil recovery (MEOR) in Baolige Oilfield. Int Biodeterior Biodegrad 127:247–253. https://doi.org/10.1016/j.ibiod.2017.12.009
Sharma N, Lavania M, Kukreti V, Lal B (2020) Instigation of indigenous thermophilic bacterial consortia for enhanced oil recovery from high temperature oil reservoirs. PLoS ONE 15(5):e0229889. https://doi.org/10.1371/journal.pone.0229889
Chen Y, Pan J, Yun Y, Zhi B, Li G, Li M, Ma T (2020) Halomonas plays a central role in the syntrophic community of an alkaline oil reservoir with alkali-surfactant-polymer (ASP) flooding. Sci Total Environ 747:141333. https://doi.org/10.1016/j.scitotenv.2020.141333
Coates J (2000) Interpretation of infrared spectra: A practical approach. In: Meyers RA (ed) Encyclopedia of analytical chemistry. Wiley, Chichester, pp 10815–10837
Dean AP, Sigee DC, Estrada B, Pittman JK (2010) Using FTIR spectroscopy for rapid determination of lipid accumulation in response to nitrogen limitation in freshwater microalgae. Bioresour Technol 101:4499–4507. https://doi.org/10.1016/j.biortech.2010.01.065
European Union Risk Assessment Report P-TERT-BUTYLPHENOL, CAS No: 98-54-4, EINECS No: 202-679-0. http://europa.eu.int
Singla A, Kumar S, Lavania M, Chhipa H, Kapardar R, Rastogi S, Lal B, Sarma PM (2017) Optimization and molecular characterization of syngas fermenting anaerobic mixed microbial consortium TERI SA1. Int J Renew Energy Dev 6(3):241–251. https://doi.org/10.14710/ijred.6.3.241-251
Zhang J, Xue Q, Gao H, Lai H, Wang P (2016) Production of lipopeptide biosurfactants by Bacillus atrophaeus 5–2a and their potential use in microbial enhanced oil recovery. Microb Cell Fact 15:168
Chrzanowski L, Dziadas M, Lawniczak L, Cyplik P, Białas W, Szulc A, Lisiecki P (2012) Biodegradation of rhamnolipids in liquid cultures: effect of biosurfactant dissipation on diesel fuel/B20 blend biodegradation efficiency and bacterial community composition. Bioresour Technol 111:328–335. https://doi.org/10.1016/j.biortech.2012.01.181
Li J, Xue S, He C, Qi H, Chen F, Ma Y (2018) Effect of exogenous inoculants on enhancing oil recovery and indigenous bacterial community dynamics in long-term field pilot of low permeability reservoir. World J Microbiol Biotechnol 34:53. https://doi.org/10.1007/s11274-018-2433-8
Wang L, Tang Y, Wang S, Liu RL, Liu MZ, Zhang Y, Liang FL, Feng L (2006) Isolation and characterization of a novel thermophilic Bacillus strain degrading long-chain n-alkanes. Extremophiles 10(4):347–356. https://doi.org/10.1007/s00792-006-0505-4
Liu QK, Wang J, Li GQ, Ma T, Liang FL, LiuR L (2008) Characterization of a thermophilic Geobacillus strain DM-2 degrading hydrocarbons. HJKX China 29(12):3554–3560
Han HY, Zhu WY, Song ZY (2017) Mechanisms of oil displacement by Geobacillus stearothermophilus producing bio-emulsifier for MEOR. Pet Sci Technol 35(17):1791–1798. https://doi.org/10.1080/10916466.2017.1320675
Xia W, Dong H, Yu L (2012) Oil-degrading characterization of thermophilic and halotolerant strain Geobacillus sp. WJ-2. J Cent South Univ 43(1):8–16
Tourova TP, Sokolova DS, Semenova EM, Shumkova ES, Korshunova AV, Babich TL, Poltaraus AB, Nazina TN (2016) Detection of n-alkane biodegradation genes alkB and ladA in thermophilic hydrocarbon-oxidizing bacteria of the genera Aeribacillus and Geobacillus. Microbiology 85(6): 693–707. Doi:https://doi.org/10.1134/S0026261716060199
Olguin-Lora P, Le Borgne S, Castorena-Cortes G, Roldan-Carrillo T, Zapata-Penasco I, Reyes-Avila J (2011) Evaluation of haloalkaliphilic sulfur-oxidizing microorganisms with potential application in the effluent treatment of the petroleum industry. Biodegradation 22:83–93. https://doi.org/10.1007/s10532-010-9378-4
Sorokin DY, Tourova TP, Kolganova TV, Detkova EN, Galinski EA, Muyzer G (2011) Culturable diversity of lithotrophic halo alkaliphilic sulfate-reducing bacteria in soda lakes and the description of Desulfonatronumthioautotrophicum sp. nov., Desulfonatronumthiosulfatophilum sp. nov., Desulfonatronovibriothiodismutans sp. nov., and Desulfonatronovibriomagnus sp. nov. Extremophiles 15(3):391–401. https://doi.org/10.1007/s00792-011-0370-7
Sorokin DY, Van den Bosch PL, Abbas B, Janssen AJ, Muyzer G (2008) Microbiological analysis of the population of extremely haloalkaliphilic sulfur-oxidizing bacteria dominating in labscale sulfde-removing bioreactors. Appl Microbiol Biotechnol 80:965–975. https://doi.org/10.1007/s00253-008-1598-8
Sorokin DY, Van Pelt S, Tourova TP, Muyzer G (2007) Microbial isobutyronitrile utilization under haloalkaline conditions. Appl Environ Microbiol 73:5574–5579
Aboelkhair H, Diaz P, Attia A (2021) Biosurfactant production using Egyptian oil fields indigenous bacteria for microbial enhanced oil recovery. J Pet Sci Eng 208:109601. https://doi.org/10.1016/j.petrol.2021.109601
ONGC TERI Biotech Limited (OTBL) https://www.otbl.co.in/Case-Studies-meor.php
Omoniyi OA, Abdulmalik F (2015) A review of microbial enhanced oil recovery: current development and future prospects. Int J Sci Eng Res 6(1):1378–1389
Acknowledgements
The authors would like to thank The Energy and Resources Institute (TERI) for providing the infrastructure facilities (Gas chromatography, anaerobic culturing facilities) for conducting the present investigation. The authors would also like to acknowledge the facility of Teri Deakin Nano-Biotechnology Center, Gurugram (FTIR, and SEM). The authors declare that this study was funded by Oil and Natural Gas Corporation Ltd (AMD/IRS/MM/MISC/03/2016-18) Institute Of Reservoir Studies, Ahmedabad.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No author has any conflict with the submitted manuscript. It was reviewed and approved by the co-authors for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sharma, N., Lavania, M., Kukreti, V. et al. Enhanced Oil Recovery Using Indigenous Microbiome of High Temperature Oil Reservoirs. Curr Microbiol 80, 179 (2023). https://doi.org/10.1007/s00284-023-03272-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00284-023-03272-6