Abstract
One of the immune responses desired to be achieved by SARS-CoV-2 vaccination is to create neutralizing antibodies (nAbs), thus preventing the development and spread of infection. The aim of this study was to investigate the seropositivity rate, anti-spike antibody levels, and neutralizing capacity of these antibodies against wild type (WT) and alpha variants in serum samples of individuals who had been naturally infected or vaccinated with CoronaVac®. Total anti-spike antibody levels were determined in all samples. Neutralization assays were performed by the reduction of the cytopathic effect in Vero-E6 cells with infectious WT and alpha SARS-CoV-2 variants.
Although both naturally infected and vaccinated individuals were all seropositive for antispike antibodies, 84.8% of the vaccinated group, and 89.3% of the naturally infected group had detectable nAbs. The nAbs titers were significantly higher in the naturally infected group for both WT and alfa variant of the virus as compared to the vaccinated individuals.
In this study, it was observed that all individuals became seropositive six weeks after exposure to the vaccine or the virus. Moreover, naturally infected individuals had higher levels of nAbs than those vaccinated. The presence of nAbs against the alpha variant in both naturally infected and vaccinated individuals suggests that these antibodies may also be protective against infections, which may be caused by other variants, such as delta and omicron.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
SARS-CoV-2, the causative agent of COVID-19 that was declared a pandemic by the World Health Organization on March 11, 2020, is a coronavirus that first emerged in China in December 2019 and spread rapidly around the world [1]. SARS-CoV-2 causes the emergence of variants that may have different characteristics with the development of mutations over time [2]. The genetic evolution of SARS-CoV-2 was minimal during the early phase of the pandemic, with the emergence of a globally dominant variant called D614G [wild-type (WT)] [2]. Upon the uncontrollable spread of SARS-CoV-2, multiple variants of SARS-CoV-2 have been described, of which a few are considered variants of concern (VOCs) given their impact on public health [2, 3]. In December 2020, routine genomic surveillance in the United Kingdom (UK) reported a new and rapidly growing lineage with an unexpectedly large number of genetic changes, including that in the receptor-binding domain of SARS-CoV-2 [variant VOC202012/01, lineage B.1.1.7 (alpha)] [4]. Reports began to appear that this new variant was rapidly spreading all over the world as of January 2021 [5, 6].
During the COVID-19 pandemic, which was characterized by high morbidity and mortality rates, vaccine development has become a global priority [3]. Various vaccines have been applied worldwide to prevent the development of COVID-19 infection via different mechanisms [7]. The SARS-CoV-2 vaccination program in Turkey started on January 11, 2021, with the application of CoronaVac® (Sinovac Life Science Co., Ltd., Beijing, China), an inactivated complete viral vaccine [8]. One of the immune responses desired to be achieved by vaccination is to create neutralizing antibodies in individuals, thus impeding the development and spread of infection by preventing the entry, fusion, or release of the virus from the cell [7]. The presence and titers of neutralizing antibodies are important for virus clearance, protection from and prediction of SARS-CoV-2 infection, and prediction of clinical outcomes of SARS-CoV-2 infection [9, 10]. Few data are available on the neutralization capacity of the CoronaVac® vaccine against the variants, and whether this vaccine produces neutralizing antibodies against different variants of SARS-CoV-2 has not been clearly resolved [8]. In the phase 2 study reports of CoronaVac® vaccine, the neutralization activity was measured earliest at the 14th day after the second dose of vaccination, and the neutralizing antibody seroconversion level was determined as 50%. However, seroconversion of neutralizing antibodies increased to 79% in experiments performed on the 28th day after the second dose [11]. However, the power of the CoronaVac vaccine in neutralizing SARSCoV-2 variants has not yet been clarified [12].
In this study, we aimed to investigate the seropositivity rate, anti-spike antibody levels, and neutralizing capacity of these antibodies against WT and alpha variants in serum samples obtained after the 6th week of PCR positivity in individuals who developed an immune response due to inadvertent SARS-CoV-2 infection and in those who were presumed to develop anti-SARS-CoV-2 antibodies after 14 days from the second dose vaccination.
Materials and Methods
Study Groups and Sample Collection
We retrospectively evaluated the titers of SARS-CoV-2 S1 receptor-binding domain (RBD) total antibodies and neutralizing antibodies (nAbs) titers against the SARS-CoV-2 WT and alpha variant in sera from uninfected persons who received two doses of CoronaVac ®, and compared these with sera from unvaccinated, naturally infected individuals.
The first study group compared individuals with SARS-CoV-2 infection with positive viral RNA in nasopharyngeal swab samples using the real-time polymerase chain reaction (RT-PCR) method between August and November 2020. These individuals were not vaccinated, and after PCR positivity, they did not receive any vaccines. Serum samples were taken in the 6th week after PCR positivity.
In the second group of our study, serum samples were collected from individuals who were immunized with CoronaVac® between January and February 2020 on the 14th day after the second dose (i.e., 6 weeks after the first dose) of vaccination. Individuals with previous or subsequent SARS-CoV-2 infection at the time of inoculation with the first vaccination dose were excluded.
Serum samples were stored at – 80 °C until the neutralizing antibody detection assay.
CoronaVac® Vaccination Protocol
The vaccine was produced from a novel coronavirus (strain CZ02) grown in kidney cell cultures of the African green monkey Vero cells and contains inactivated SARS-CoV-2 virus, aluminum hydroxide, disodium hydrogen phosphate dodecahydrate, sodium dihydrogen phosphate monohydrate, and sodium chloride. A single dose of 0.5 mL contains 600 SU of inactivated SARS-CoV-2 virus as antigen. The vaccinated participants received two doses of inactive CoronaVac® (Sinovac Life Science Co, Ltd, Beijing, China) vaccine. The second dose was administered 28 days after the first dose as recommended. The vaccine was administered intramuscularly in the deltoid region of the upper arm [13].
Detection of SARS-CoV-2 RNA
SARS-CoV-2 genomic RNA was detected in the nasopharyngeal samples of patients with COVID-19 infection using a Bio-speedy SARS-CoV-2 (2019-nCoV) RT-qPCR detection kit (Bioeksen, Istanbul, Turkey) and a LightCycler® 480 Instrument II (Roche Molecular Systems, USA), using the reverse transcription RT-PCR method.
Detection of anti-SARS-CoV-2 Antibodies
Electrochemiluminescent Immunoassay
The total antibody levels developed against the spike protein were determined in all samples using an Elecsys anti-SARS-CoV-2 S Quantitative kit (Roche Diagnostics International AG. Rotkreuz, Switzerland) kit according to the manufacturer’s instructions. The assay uses a recombinant protein representing the RBD of the spike antigen in a double-antigen sandwich electrochemiluminescence immunoassay, which uses streptavidin-coated microparticles to separate bound from unbound substances prior to applying voltage to the electrode. This assay detects total antibodies (predominantly IgG, IgA, and IgM) against an epitope of the viral spike protein RBD. Samples were prepared according to the manufacturer’s instructions and analyzed using a Roche Cobas e601 analyzer. In the first step, 20 µL of sample, biotinylated SARS-CoV-2 S-RBD-specific recombinant antigen, and SARS-CoV-2 S-RBD-specific recombinant antigen labeled with a ruthenium complex formed a sandwich complex. In the second step, after the addition of streptavidin-coated microparticles, the complex was bound to the solid phase via the interaction between biotin and streptavidin. The mixture was then aspirated into the measuring cell, where microparticles were magnetically captured onto the surface of the electrode. The application of a voltage to the electrode then induced chemiluminescent emission, which was measured using a photomultiplier. The results obtained at the end of these processes were determined via a calibration curve, which is an instrument-specifically generated 2-point calibration and a master curve provided via the reagent barcode. Using the manufacturer’s guidelines, a cut-off value of ≥ 0.8 U/mL was accepted as positive. Dilutions were performed on specimens with values greater than 250 U/mL according to the manufacturer’s guidelines [14].. The Ggreenhouses that were measured with antibodyies titers above 250 were diluted and studied again. When interpreting the results, antibody titers were grouped as 1–125, 126–250, and >250 U/mL, and evaluated in percentages.
Neutralizing Antibody Detection
To assess the presence of neutralizing SARS-CoV-2 antibodies, blood samples from all the participants were analyzed. Neutralization assays were performed essentially by the reduction of cytopathic effect (CPE) in Vero E6 cells with infectious D614G (wild type, WT) (GISAID Accession Number EPI_ISL_3509539), and B.1.1.7 (Alpha) (GISAID Accession Number EPI_ISL_1167921) SARS -CoV-2 variants.
The previously described virus neutralization assay procedures were followed [15].
Briefly, Vero E6 cells (3.3x104 per well) were seeded 24 h before infection in a 96-well plate (TPP Techno Plastic Products AG, dTrasadingen, Switzerland). Neutralization assays were performed by incubating serial dilutions (1:4 to 1:2048) of 100 µL of heat-inactivated serum samples with 100 µL (100 TCID50) of each variant. The mixture of virus Ab mix (200 µL) was then added to the cells in 96-well plates, and the plates were incubated at 37˚C for three days incubation to develop a full CPE as determined by microscopic examination. The highest dilution of serum that showed full CPE inhibition was recorded as the nAbs titer. All samples were tested in duplicates. On each 96-well plate, Ab-positive and -negative serum samples and no-serum-added wells, as well as no-virus-added wells, were included. Only at 1/2 serum dilutions were the samples toxic to cells, and in the remainder of the assays, the samples were diluted starting at 1/4 dilution.
Statistical Analysis
Statistical Package for the Social Sciences version 23.0 (IBM Corp.) was used to analyze the data. Descriptive statistics, including frequencies and means, for all variables were calculated. The results were expressed as mean ± standard deviation, median, range (minimum– maximum), and percentage (%). The Shapiro-Wilk test was used to examine whether the numerical variables showed a normal distribution. A Chi-square test or Fisher’s exact test was used for the comparison of categorical variables, and Mann-Whitney U test was used to investigate differences between the two independent groups in terms of binary variables. Oneway analysis of variance was used to compare more than two independent groups.
Spearman’s correlation coefficient was used to determine whether there was a significant relationship between binary variables. The level of statistical significance was set at p < 0.05.
Results
In the first group of enrolled 28 individuals [overall mean age (years) (mean ± SD) 43 ± 15.42 (range 22 to 79, > 65, 10.7%)] with a confirmed SARS-CoV-2 quantitative RT-PCR test. Disease severity in all patients was mild to moderate according to the World Health Organization (WHO) criteria, and serum samples were taken six weeks after PCR positivity [16]. In the second group of 33 individuals [overall mean age (year) (mean ± SD) 42.61 ± 9.69 (range 22 to 62)] who received two doses of the CoronaVac® vaccine. The first doses of the vaccine were administered from January 11 to January 18, 2021. The second dose was administered 28 days after the first dose. Sera were drawn 14 days after the second dose of vaccination to evaluate the antibody levels and neutralization potency. Demographics, clinical characteristics, and antibody levels of naturally infected COVID-19 patients and CoronaVac®-vaccinated individuals are shown in Table S1 and S2.
A total of 61 participants were included in our study, of which, 30 were male (49.2%) and 31 were female (50.8%). Of the CoronaVac® vaccinated patients, 14 were male (42.4%), 19 were female (57.6%), while in the group who were naturally infected (Group 2), there were 16 males (57.1%) and 12 females (42.9%). There were no statistically significant differences between the two groups in terms of sex and age (P = 0.252 and P = 0.907, respectively).
We found that 100% of individuals were seropositive for spike protein specific total antibodies. Although not statistically significant, total anti-S SARS-CoV-2 antibody levels of individuals in our study were higher in women [median (IQR) = 163.1 (56.3–404.9)] than in men [median (IQR) = 125.8 (69.65–265.67)] (P = 0.634). SARS-CoV-2 total anti-S antibody levels based on age and sex in naturally infected and vaccinated individuals are shown in Table 1.
Among vaccinated individuals, higher levels of anti-S were measured when compared to the elderly (P = 0.015), regardless of whether total anti-S levels increased with age in the naturally infected group, but no statistically significant difference was found (P = 0.149) (Table 1).
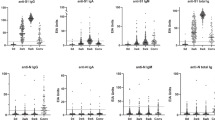
Neutralizing antibodies against WT and alpha variant virus were undetectable (or below the limit of detection < 1/4) in 13.1% (8/61) and 8.2% (5/61) of serum samples, respectively (Figure 1). In the CoronaVac® vaccinated group, 84.8% had a titer of 1/4 or more of nAbs, and higher levels of nAbs were observed against the alpha variant compared to WT. However, there was no difference in nAbs titer between WT and alpha variants, as well as the presence of 89.3% nAbs in the naturally infected group (Figure 1, Table 2). Correlation analysis showed a positive correlation between nAbs titers against WT and alpha variants and anti-S antibody levels in naturally infected subjects (rho = 0.565, P = 0.002; rho = 0.559, P = 0.001, respectively). Furthermore, a positive correlation was found between nAbs titers and anti-S antibody levels against the WT and alpha variants in vaccinated individuals (rho = 0.709, P < 0.001; rho = 0.718, P < 0.001, respectively). Considering the relationship between the level of nAbs hyphens and the age of the individuals, there was no statistically significant difference in naturally infected individuals over the age of 40 years, but higher titers of nAbs were formed against both WT and alpha variants. Although no significant difference was detected in individuals over 40 years of age in the vaccinated group, a higher rate of nAbs was found against the alpha variant (Table 3).
Distrubution of neutralizing antibody titers of 61 CoronaVac®-vaccinated and naturally infected individuals. Neutralizing antibody content of sera samples from 33 CoronaVac®-vaccinated and 28 naturally infected individuals were analyzed using SARS-CoV-2 variants [wild type (D614G, lineage B), and alpha (lineage B.1.1.7)]. nAbs titers were significantly high in naturally infected individuals than vaccinated ones against both wild type (P = 0.003) and alpha variant virus (P = 0.006), respectively. Statistical significance was determined using the two‐tailed Mann–Whitney U test. p values < 0.05 were considered significant. GMTs and 95% CIs are indicated: Wild type (9.51, 32.90), alpha (17.55, 47.73) variant. CI, confidence interval; GMT, geometric mean titer
Discussion
Elucidation of the magnitude of the SARS-CoV-2 antibody response greatly contributes to the assessment of the effectiveness of vaccination and also helps to better understand COVID-19 pathogenesis as well as the development of potential therapeutic agents and vaccines [17]. Although the presence of antibodies is accepted as an important indicator of the immune response, it does not imply protection [18]. The presence and level of nAbs are the main indicators to show protection [18, 19]. In our study, seropositivity rates, antibody levels, and neutralizing antibodies were examined at the 6th week after PCR positivity following natural infection and on the 14th day after the second dose (i.e., at 6 weeks after the first dose) vaccination in individuals.
Seropositivity is one of the parameters that stimulates the immune system upon exposure to an infectious agent or vaccination [18]. In our study, all individuals became seropositive six weeks after exposure to the vaccine or virus. In phase 1–2 study of the CoronaVac vaccine conducted in China, the positivity for anti-S antibodies was found to be 100% after two dosages administered 28 days apart, while this rate was found to be 89.7% in a phase 3 study conducted in Turkey [20].
Anti-S antibody seropositivity rates after the second dose of Coronavac® vaccine in uninfected individuals in Turkey were reported to be 99.6% and 100% by two studies [21, 22]. Seropositivity rates vary among naturally infected individuals. Zhao et al. [23] reported that while anti-S antibody positivity was 100% within 15–39 days after the onset of the disease, Liu et al. [24] determined this rate as 85.7% in the sixth week of the disease.
One of the most important parameters affecting antibody response is age [18]. Due to the change in T cell function and decrease in B lymphocyte production with increasing age in adults, level of the antibody response after infection or vaccination in older may be lower than that in young people, or no antibodies may develop [25]. In various studies performed after influenza, hepatitis A, hepatitis B, pneumococcal, tick-borne encephalitis, tetanus, and SARSCoV-2 vaccination, it was observed that the post-vaccine antibody response was inversely proportional to age, and antibody titers decreased over time [17, 25]. In our study, similar to the literature, it was determined that among vaccinated individuals, young people produced statistically significantly higher levels of anti-S antibodies than the elderly. In contrast, anti-S antibody levels were found to be proportionally higher, albeit not statistically significant, in individuals over the age of 40 years in the group with natural infection.
Antibodies that inhibit viral attachment are called “neutralizing antibodies [19]”. nAbs against SARS-CoV-2 are formed against RBD in the “spike (S)” protein [11]. Conventionally, the gold standard method for evaluating the presence and neutralization potential of these antibodies is the “plaque reduction neutralization test (PRNT) [19]”. In China, the nAbs seropositivity rates of the CoronaVac® vaccine were 97–100% for the WT variant in phase 1 and 2 studies, whereas the formation of neutralizing antibodies was 98–99% in volunteers over 60 years of age [11, 26]. In a phase 3 study conducted in our country, the presence of nAbs were detected at a rate of 92% in a group of 356 people [20]. Similar to Tanriover et al. [20], in our study, it was found that 86.9% of the individuals had nAbs against WT and 91.8% against the alpha variant. In addition, although there was no statistically significant difference in naturally infected individuals over 40 years of age compared to individuals under 40 years of age, higher titers of nAbs were detected against both the WT and alpha variants.
Fernández et al. [8] in Chile and Vacharathit et al. [27] in Thailand reported that the titers of the nAbs response against alpha variants were significantly lower than those of the WT after two-dose vaccination with CoronaVac®. In a study by Chen et al. [28], sera were equally effective in neutralizing the WT and alpha variants in individuals vaccinated with CoronaVac®. In our study, naturally infected individuals had higher levels of nAbs than those vaccinated with CoronaVac®. While there was no difference between WT and alpha variants in nAbs titers developed in naturally infected individuals, in our study, contrary to the findings of the studies by Fernández et al. [8], Vacharathit et al. [27], and Chen et al. [28], the titers of the nAbs response against alpha variants were higher than those against the WT variant in two dose-vaccinated CoronaVac strains.
The present study has some limitations. First, the study was conducted at a single center. Second, the fact that individuals with asymptomatic infections could not be detected in the period between vaccination and sampling may have a small effect on the results, as the distinction between vaccine-elicited antibodies and antibodies originating from natural infection will be affected.
Conclusions
In our study, the presence of nAbs against the alpha variant in both naturally infected with WT and uninfected CoronaVac®-vaccinated individuals suggests that these antibodies may also protect from the infections caused by other variants, such as delta and omicron. In addition, the presence of limited data on the neutralizing power of the CoronaVac® vaccine against different variants reveals that large-scale studies should be conducted to determine vaccine dosing applications that will create a long-term nAbs memory response.
Data Availability
Data sharing is not applicable to this article, as no new data were created or analyzed in this study.
References
Lei Q, Li Y, Hou HY et al (2021) Antibody dynamics to SARS-CoV-2 in asymptomatic COVID-19 infections. Allergy 76(2):551–561. https://doi.org/10.1111/all.14622
Aleem, A., Akbar Samad, A. B., & Vaqar, S. (2023). Emerging Variants of SARS-CoV-2 And Novel Therapeutics Against Coronavirus (COVID-19). In StatPearls. StatPearls Publishing
Estofolete CF, Banho CA, Campos GRF et al (2021) Case study of two post vaccination SARS-CoV-2 infections with P1 variants in CoronaVac vaccinees in Brazil. Viruses 13(7):1237. https://doi.org/10.3390/v13071237
Rambaut A, Loman N, Pybus O, et al. Preliminary genomic characterisation of an emergent SARS-CoV-2 lineage in the UK defined by a novel set of spike mutations (2020) https://virological.org/t/preliminary-genomic-characterisation-of-an-emergent-sars-cov-2-lineage-in-the-uk-defined-by-a-novel-set-of-spike-mutations/563
O’Toole Á Tracking the international spread of SARS-CoV-2 lineages B.1.1.7 and B.1.351/501Y-V2” (2021); http://virological.org/t/tracking-the-international-spread-of-sars-cov2-lineages-b-1-1-7-and-b-1-351-501y-v2/592
Davies NG, Abbott S, Barnard RC et al (2021) Estimated transmissibility and impact of SARS-CoV-2 lineage B117 in England. Science 372(6538):eabg3055. https://doi.org/10.1126/science.abg3055
Zhao J, Zhao S, Ou J et al (2020) COVID-19: coronavirus vaccine development updates. Front Immunol 23(12):11–602256. https://doi.org/10.3389/fimmu.2020.602256
Fernández J, Bruneau NFASCE, Fasce R, Martín HS, Balanda M, Bustos P, Ulloa S, Mora J, Ramírez E (2022) Neutralization of alpha, gamma, and D614G SARS-CoV-2 variants by CoronaVac vaccine-induced antibodies. J Med Virol 94(1):399–403. https://doi.org/10.1002/jmv.27310
Khoury DS, Cromer D, Reynaldi A et al (2021) Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med 27(7):1205–1211. https://doi.org/10.1038/s41591-021-01377-8
Morales-Núñez BJJ, Muñoz-Valle JF, Torres-Hernandez PC (2021) Overview of neutralizing antibodies and their potential in COVID. J Vaccines (Basel) 9(12):1376. https://doi.org/10.3390/vaccines9121376
Zhang Y, Zeng G, Pan H et al (2021) Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis 21(2):181–192. https://doi.org/10.1016/S1473-3099(20)30843-4
Riester E, Findeisen P, Hegel JK et al (2021) Performance evaluation of the Roche Elecsys Anti-SARS-CoV-2 S immunoassay. Journal of Virological Methods 297:114271. https://doi.org/10.1016/j.jviromet.2021.114271
https://www.covidvaccine.gov.hk/pdf/CoronaVac_ENG_PI_brief.pdf
Elecsys® anti-SARS-CoV-2 assay method sheet; 09203095501, V.9.0. https://www.fda.gov/media/137605/download
Shen C, Wang Z, Zhao F et al (2020) Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA 323(16):1582–1589. https://doi.org/10.1001/jama.2020.4783
World Health Organization (2020) Clinical management of COVID-19 patients interim guidance. World Health Organization, Geneva, Switzerland
Uysal EB, Gümüş S, Bektöre B, Bozkurt H, Gözalan A (2022) Evaluation of antibody response after COVID-19 vaccination of healthcare workers. J Med Virol 94(3):1060–1066. https://doi.org/10.1002/jmv.27420
Zimmermann P, Curtis N (2019) Factors that influence the immune response to vaccination. Clin Microbiol Rev 32(2):e00084-18. https://doi.org/10.1128/CMR.00084-18
Şenol Akar Ş, Akçalı S, Özkaya Y et al (2021) Factors affecting side effects, seroconversion rates and antibody response after inactivated SARS-CoV-2 vaccination in healthcare workers. Mikrobiyol Bul 55(4):519–538. https://doi.org/10.5578/mb.20219705
Tanriover MD, Doğanay HL, Akova M et al (2021) Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet 398(10296):213–222. https://doi.org/10.1016/S0140-6736(21)01429-X
Yalçın TY, Topçu DI, Doğan Ö et al (2022) Immunogenicity after two doses of inactivated virus vaccine in healthcare workers with and without previous COVID-19 infection: prospective observational study. J Med Virol 94(1):279–286. https://doi.org/10.1002/jmv.27316
Bayram A, Demirbakan H, Günel Karadeniz P, Erdoğan M, Koçer I (2021) Quantitation of antibodies against SARS-CoV-2 spike protein after two doses of CoronaVac in healthcare workers. J Med Virol 93(9):5560–5567. https://doi.org/10.1002/jmv.27098
Zhao J, Yuan Q, Wang H et al (2020) Antibody responses to SARS-CoV-2 in patients with novel coronavirus disease 2019. Clin Infect Dis 71(16):2027–2034. https://doi.org/10.1093/cid/ciaa344
Liu ZL, Liu Y, Wan LG, Xiang TX, Le AP, Liu P, Peiris M, Poon LLM, Zhang W (2020) Antibody profiles in mild and severe cases of COVID-19. Clin Chem 66(8):1102–1104. https://doi.org/10.1093/clinchem/hvaa137
Weinberger B, Grubeck Loebenstein B (2012) Vaccines for the elderly. Clin Microbiol Infect 18(Suppl 5):100–108. https://doi.org/10.1111/j.1469-0691.2012.03944.x
Wu Z, Hu Y, Xu M et al (2021) Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine (CoronaVac) in healthy adults aged 60 years and older: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis 21(6):803–812. https://doi.org/10.1016/S1473-3099(20)30987-7
Vacharathit V, Aiewsakun P, Manopwisedjaroen S et al (2021) CoronaVac induces lower neutralising activity against variants of concern than natural infection. Lancet Infect Dis 21(10):1352–1354. https://doi.org/10.1016/S1473-3099(21)00568-5
Chen Y, Shen H, Huang R, Tong X, Wu C (2021) Serum neutralising activity against SARS-CoV-2 variants elicited by CoronaVac. Lancet Infect Dis 21(8):1071–1072. https://doi.org/10.1016/S1473-3099(21)00287-5
Acknowledgements
The authors would like to thank the editor and reviewers of the journal for their contributions.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Concept, Design: EÖ, IB, İT, CKB, NK, FA. Data collection and/or processing: EÖ, MY IB, NSÇ, İT, CKB, MZD. Analysis and/or interpretation: EÖ, MY, IB, İT, CKB, NSÇ, MZD. Writing manuscript: EÖ, MY IB, NSÇ, İT, NK, FA, MZD.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Ethical Approval
This study was approved by the local ethics committee of the Medical School of Karadeniz Technical University (date: November 2021; protocol no:2021/305) and the COVID-19 Scientific Research Evaluation Committee of the General Directorate of Health Care Services of the Ministry of Health of the Republic of Turkey.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Özkaya, E., Yazıcı, M., Baran, I. et al. Neutralization of Wild-Type and Alpha SARS-CoV-2 Variant by CoronaVac® Vaccine and Natural Infection- Induced Antibodies. Curr Microbiol 80, 162 (2023). https://doi.org/10.1007/s00284-023-03248-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00284-023-03248-6