Abstract
Infectious diseases remain one of the major health challenges worldwide due to the problem of antimicrobial resistance. Conventional antimicrobials have the disadvantage that bacteria rapidly acquire resistance to them, so alternatives must be developed to combat antibiotic resistance. Nanotechnology and the repurposing of existing drugs with known biological profiles are new approaches to replacing conventional antimicrobials. In this paper, we have tested the antibacterial activity of sodium acetate (NaA), vitamin C (VC), and zinc oxide nanoparticles (ZnO NPs) against Escherichia coli O157:H7 ATCC 51659 and Pseudomonas aeruginosa ATCC 27853. MIC values for tested compounds ranged from 0.08 to 6.5 mg ml−1, and the effect of combinations and safety profiles against HepG2 cell line of these compounds were also evaluated. At sub-MIC values, tested compounds had a potential antivirulence effect by inhibiting motility and reducing biofilm formation and maturation. Collectively, ZnO NPs and VC are considered safe alternatives to traditional antibiotics that are capable of reducing the development of antibiotic resistance in microbes.
Graphical Abstract

Graphical abstract representing the main aim and the final findings of our work. Spread of multidrug-resistant (MDR) bacterial strains created an urge for alternative safe antimicrobial agents. In this work, we found that ZnO NPs and vitamin C are potential candidates that could be used against MDR E.coli and P. aeruginosa.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The discovery of antibiotics is considered the most significant achievement known to man in the modern era. Despite having a limited spectrum and nature, they contributed to saving many lives during World War II [1]. Unfortunately, bacteria have developed and acquired strategies to resist antibiotics. In turn, this has led to the inevitability of the rapid development of known antibiotics and the development of novel antibiotics that require time, effort, and millions of dollars, creating an additional economic burden [2].
Infections with drug-resistant bacteria have increased morbidity and mortality, hospitalization length, and care costs. Antimicrobial drug resistance is on the rise and is currently responsible for more than 700,000 deaths per year worldwide, according to a report from the World Bank in 2017 [3]. According to WHO reports, it is estimated that 300 million people will die prematurely because of infections with multidrug-resistant organisms over the next 35 years [4, 5]. In addition, the world can expect to lose 60–100 billion US dollars in economic output if antimicrobial drug resistance is not effectively tackled [6].
Therefore, finding different strategies to combat bacterial resistance to antibiotics has become necessary. There are many alternative methods, including nanotechnology, extraction of new antibacterial agents from natural products [7] or the repurposing some safe and FDA-approved drugs or chemicals and testing their efficacy as antibacterial and antivirulence activities [8,9,10].
One of these alternative methods is nanotechnology [11, 12], in which tiny nanoparticles can target multiple structures in bacteria, making the development of bacterial resistance impossible, Recent studies focused on developing new methods to synthesize effective, safe formulas of nanoparticles either alone or combined with other elements [13, 14]. We are interested in metal and metal oxide nanoparticles as they have superior advantages over other types [15], e.g., reduced size, controlled shape, broad antimicrobial spectrum, and toxicity to multiple bacterial structures; thus, it is challenging for pathogens to develop resistance to metal and metal oxide nanoparticles [16].
On the other hand, repurposing already-approved drugs is another time and effort-saving strategy. Natural and FDA-approved synthetic compounds were tested for their potential antimicrobial and antivirulence activities [17,18,19]. This approach aims to disarm bacteria by disabling virulence determinants such as motility, adhesion, biofilm formation, and invasion [8, 20].
Gram-negative species are ubiquitous and associated with many infections at different body sites. In addition, they are intrinsically resistant to many antibiotics [21]. Antimicrobial resistance (AMR) in Gram-negative bacteria has become increasingly problematic for healthcare systems over the past years, with escalating costs and mortality [22]. Gram-negative species are frequently associated with nosocomial infections, including bloodstream infections, hospital-acquired pneumonia, urinary tract infections, skin and soft-tissue infections, and complicated intra-abdominal infection [23].
The current work aimed to evaluate the antibacterial and the potential antivirulence activity of ZnO NPs alone or combined with natural substances with known antimicrobial activities, e.g., vitamin C and sodium acetate against Escherichia coli O157:H7 ATCC 51659 (E. coli) and Pseudomonas aeruginosa ATCC 27853 (P. aeruginosa).
Material and Methods
Bacterial Strains, General Growth Conditions
In this study, E. coli and P. aeruginosa standard strains were employed, and E. coli O157:H7 ATCC 51659 was purchased from Egyptian Microbial Culture Collection (EMCC), Faculty of agriculture, Ain Shams University, while P. aeruginosa ATCC 27853 was a gift from Department of Microbiology and Immunology, Faculty of Pharmacy, Tanta University, Egypt. Bacterial strains were grown in Luria–Bertani (LB) at 37° C unless specified elsewhere.
Reagents
Reagents used in the present study were purchased from Biolab-European Union, Sigma/Aldrich—USA, and Oxoid-UK. All reagents and chemicals used throughout the study were ≥ 99% pure.
Experimental Design and Statistical Analysis
In this work, we aimed to test the antimicrobial activity of ZnO NPs, VC, and NaA on standard bacterial strains; the potential activity was determined by disk diffusion method for the tested substances versus ofloxacin as a reference standard. MIC for promising substances was determined, then cytotoxicity and antivirulent activity were determined. All experiments were conducted in triplicates. Statistical analysis was performed using OriginPro 2019b (OriginLab, Massachusetts, USA).
Zinc Oxide Nanoparticles
Zinc oxide nanoparticles (ZnO NPs) used in this study were prepared by wet chemical method as previously described [24]. The prepared nanoparticles were characterized for size and stability using UV–Vis spectrophotometry (T80 + PG instrument) and FEI Tecnai G2 F20 X-TWIN Transmission Electron Microscope (TEM).
Investigation of the Antibacterial Activity of Tested Compounds
To evaluate the antibacterial activity of sodium acetate (NaA), vitamin C (VC), and ZnO NPs, the standard Kirby-Bauer disk diffusion method was used [25]. Briefly, the overnight cultures of the tested E. coli and P. aeruginosa strains were diluted to a starting concentration of 1 × 108 CFUml−1, and 100 µl of microbial suspensions was spread onto the surface of Muller Hinton agar plates, pH 7.2.
Sterilized filter paper disks with a standard amount of the tested compound (10 µl of 0.82–8.2 mg ml−1 of NaA, 20–100 mg ml−1 VC and 0.2–1 mg ml−1 of ZnO NPs) were then applied to the surface. Plates were then incubated at 37° C overnight. The diameters of the zones of inhibition were measured. Ofloxacin (OFX) was used as reference antibiotic.
Determination of Minimum Inhibitory Concentrations for the Tested Compounds
The minimum inhibitory concentrations (MICs) of tested compounds were determined using the standard microbroth twofold serial dilution technique following the standard procedures of CLSI [26]. Briefly, overnight cultures of the tested bacterial strains were diluted to a final concentration of 1.5 × 105 CFUml−1, and the tested concentrations ranged from 15.6–1, 1.28–8.2, and 0.0156–10 mg ml−1 of VC, NaA, and ZnO NPs, respectively. The cultures were then incubated under shaking (~ 150 rpm) at 37° C, and the results were evaluated by measuring the optical density at 600 nm [27]. Ofloxacin (OFX) was used as reference antibiotic. All experiments were conducted in triplicates.
Evaluating the Effect of the Combination of the Tested Compounds
The combined effect of the VC or NaA with ZnO NPs was evaluated by checkerboard method to obtain the fractional inhibitory concentration (FIC) index [28, 29]. Across the column, each well contained the same concentration of ZnO NPs and then diluted along the x-axis. While in the rows, each well contained the same concentration of VC or SA that was diluted along the y-axis of a 96-well plate.
Inhibition of Biofilm Formation
In this assay, 106 Cfuml−1 of P. aeruginosa was incubated in 96-well microtiter plates at subinhibitory concentrations (Sub-MIC) ranging from 0.01 to 0.06 mg ml−1 of ZnO NPs, 0.82- 5 mg ml−1 of NaA, and 1–6 mg ml−1 of VC. Following 24-h incubation at 37° C, planktonic bacteria are rinsed away, and the remaining adherent bacteria (biofilms) are stained with crystal violet dye, thus allowing visualization of the biofilm using absorbance microplate reader (Biotek Elx-808) at 590 nm, the intensity of color is a function of bacterial attachment to the plate and forming a biofilm [30, 31].
Electron microscope imaging (FEI Tecnai G2 F20 X-TWIN Transmission Electron Microscope (TEM)) was used to the study the change in the structure of the formed biofilm. Briefly, a grid/ chip was used to support the formed biofilm that was then washed with phosphate-buffered saline (PBS pH = 7.2); for fixation purpose, the biofilm was treated with 2.5% glutaraldehyde followed by 50, 70, and 100% ethanol and left for air drying.
Motility Assay
Motility plates were used to quantify the swimming ability of E. coli treated with Sub-MIC of tested compounds as specified above in crystal violet assay. Motility plates were prepared using LB broth containing 0.25% of Bacto-agar, sub-MIC of ZnO NPs (0.005:0.06 mg ml−1) and (1: 4 mg ml−1) of VC were added to each plate, then 1 μl overnight culture of the strain of interest was spotted on to the center of the plate and incubated for 6–8 h. The plates were imaged, and the diameters of the ring formed by motile cells were measured to reflect the swimming ability of cells [32].
Cytotoxicity Testing
To investigate the cytotoxic effect of tested compounds, HepG2 cells have been employed using the MTT (3-[4,5-dimethylthiazole-2-yl]-2,5- diphenyltetrazolium bromide) cell viability assay, the experiment was conducted at VACSERA Co, Egypt. Briefly, cells (0.5 × 105 cells/ well), in serum-free media, were plated in a flat bottom 96-well microplate and treated with 20 µl of different concentrations of the tested compounds for 48 h at 37º C, in a humidified 5% CO2 atmosphere. After incubation, media were removed and 40 µl MTT solution/well was added and incubated again for 4 h, followed by photometric determination of the absorbance at 570 nm using microplate ELISA reader [33, 34]. Experiment was repeated in triplicate.
Results
As part of our efforts to resolve the issue of antibiotic resistance, we evaluated the antimicrobial and virulence determinants inhibiting the activity of different chemical compounds so that they might be substitutes for conventional antibiotics.
ZnO NPs, Acetate, and Vitamin C are Promising safe Antibacterial Agents
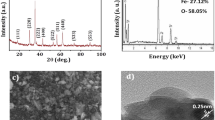
The prepared ZnO NPs were characterized for size and shape as shown in (Fig.S1). ZnO NPs showed a spherical shape and a size of approximately 50 nM. The disk diffusion technique evaluated the antibacterial potential of ZnO NPs, sodium acetate (NaA), and vitamin C (VC) against standard strains of E. coli O157:H7 and P. aeruginosa.
The results of Kirby-Bauer disk diffusion illustrated in (Table 1) revealed the bactericidal activity of ZnO NPs, NaA, and VC, and the diameters of zones of inhibition were a function of concentration; the diameter increased with increasing the concentration of the tested substance. Zones of inhibition for E. coli and P. aeruginosa were (15, 17 mm), (23, 27 mm), and (23, 25 mm) at 1, 8.2, and 100 mg ml−1 of ZnO NPs, NaA, and VC, respectively.
The broth microdilution technique was used to determine the MICs values for the tested compounds. The results are shown in Table 2. MICs of ZnO NPs, NaA, and VC for E. coli and P. aeruginosa were (0.1, 0.08 mg ml−1), (5- 3.3 mg ml−1), and (6–8 mg ml−1), respectively.
We thought to test the effect on MIC values when combining these substances. The effect of the combination of ZnO NPs, NaA, and VC was determined using a checkerboard assay. The fraction inhibitory concentration (FIC) for ZnO NPs at a constant VC concentration of 1 mg ml−1 was 0.317 and for ZnO NPs at a constant NaA concentration of 0.82 mg ml−1 was 0.27. Interestingly, MIC values of ZnO NPs for E. coli and P. aeruginosa decreased by 10 and 6.7-folds when combined with 0.82 and 1 mg ml−1 of NaA and VC, respectively, as shown in (Table. 2).
ZnO NPs, NaA, and VC were evaluated by MTT assay using HepG2 cell lines, and this showed that a significant increase in cell viability was seen with increasing ZnO NPs and in combined ZnO NPs/VC as shown in (Fig. 1). The viability of cells treated with VC and NaA around the MIC values was 90% for VC and 50–55% for NaA. We did not further study NaA as the viability of cells was low around the MIC. Furthermore, it decreased with an increase in concentration.
Cytotoxicity of ZnO NPS. ZnO/VC and NaA against HepG2 cell lines. ZnO NPs are safe at MIC value & showed increased cell viability as a function of concentration, besides cell proliferation increased in ZnO/VC combination. In NaA, safety is inversely proportional to concentration, and it showed 70% cell viability around its MIC. Error bars denote the standard deviation from three replicates
ZnO NPs and Vitamin C Substantially Inhibit Virulence Determinants in Bacteria
We assessed the potential antivirulence activity of ZnO NPs and VC by measuring the inhibition of motility and biofilm formation. A motility plate assay was used where 1 µl of E. coli O157:H7 suspension was spotted in the center of the motility plate supplemented with sublethal concentrations of ZnO NPs and VC. The diameters of motility zones decreased by increasing the concentration, as shown in (Fig. 2).
Motility assessment for E. coli O157:H7 treated with varying concentrations of ZnO NPs. a: negative control (untreated E. coli), b–d: represent increasing concentrations of ZnO NPs (5–30–60 µg ml−1), respectively. Motility zone inhibited as a function of increasing the concentration of ZnO NPs. Motility plate assay was conducted in triplicate
To further elucidate the antivirulence activity of ZnO NPs and VC, the effect of tested compounds on biofilm formation was examined. This was done to determine whether biofilms protect against target compounds. Crystal violet microtiter plate assay was used to study biofilm formation by P. aeruginosa. This method allows for the observation of bacterial adherence to an abiotic surface. Absorbance at (590 nm) for bacteria treated with Sub-MIC ZnO NPs or VC (Fig. 3) decreased as a function of increasing the tested concentrations.
Furthermore, electron microscope imaging was used for untreated and treated P. aeruginosa at sub-MIC of ZnO NPs to confirm the inhibition of biofilm production (Fig. 4). The intensity of biofilm maturation was inhibited, and planktonic cells were more predominant in treated cells.
Discussion
The problem of antimicrobial resistance is rapidly becoming a worldwide health crisis. Antimicrobial drugs have been widely used in human medicine for more than 70 years, either as prophylactics or as therapeutics, with tremendous benefits to human health. Unfortunately, the widespread and inappropriate use of antimicrobial drugs has resulted in the emergence of increasing numbers of drug-resistant microbes [35].
If strong measures are not taken to address this most urgent health problem, antimicrobial drug resistance is estimated to cost approximately 10 million lives annually by 2050 worldwide, which is more than cancer and diabetes combined [36].
In this research paper, we aimed to find alternative approaches to combat the problem of antibiotic resistance, specifically in Gram-negative bacteria. We continued our studies on ZnO NPs as potential candidates. We have tested its antimicrobial activity before [37] Here, we evaluated its antivirulence activity alone or combined with other natural substances and chemicals.
Recent studies revealed the antimicrobial and antioxidant activity of vitamin C (VC) and sodium acetate (NaA) [17, 18, 38] against a broad range of bacterial and fungal species in a concentration-dependent manner. This was the impetus to repurpose VC and NaA, evaluate their potential antivirulence activities, determine possible synergism, and their cytotoxic effects at MIC values alone or combined with ZnO NPs against the highly virulent and rapidly developing resistance E. coli and P. aeruginosa strains.
Disk diffusion and microbroth dilution methods were employed to assess the antibacterial potential of the tested substances, considering the acidity of both VC and NaA. Hence, we measured the activity at pH 4–5. The effect of pH was minor on bacterial growth with VC, but it was drastic with NaA. We fixed the pH in all experiments at pH = 7.2 using buffered media.
Our results support previous studies examining the effect of VC and acetate-containing compounds against Gram-negative bacteria like Klebsiella or carbapenem-resistant Gram-negative strains [19, 39, 40], we tested the antibacterial potential of both VC and NaA against the standard bacterial strains E. coli and P. aeruginosa. MIC values were (0.1, 0.08 mg ml−1), (5–3.3 mg ml−1) and (6–8 mg ml−1), for ZnO NPs, NaA, and VC for E. coli and P. aeruginosa, respectively. Interestingly, the tested substances showed synergistic activities when combined as the FIC for the tested substance in combination was < 0.5 [41] and the MIC values of ZnO NPs for E. coli and P. aeruginosa were reduced by 7–10-folds at fixed concentrations of VC or NaA.
The early detection of the hepatocellular toxicity of newly developed drugs is an essential step during clinical phases of drug approval [42, 43]. Therefore, cytotoxicity of the compounds was evaluated against HepG2 cell lines, and this revealed that both ZnO NPs and ZnO NPs/VC combination were safe around the MIC values where cell viability increased with increasing the concentration. NaA is physiologically present in the small intestine in concentrations of (20–40 mM), and recent studies claimed that NaA acts as a signal to initiate bacterial invasion of small intestine [44, 45]. Increasing the concentrations of NaA above the physiological values could be toxic to the bacteria [46], that’s why NaA was toxic to cells around its MIC values.
Numerous studies showed the regulation and coordination between the expression of virulence determinants and the ability of bacteria to cause disease [44, 47]. Hence, studying the antivirulence effect as well as the antibacterial effect for any promising compounds is necessary. Bacteria can adhere to surfaces and form communities encased in a slime composed of proteins, extracellular polysaccharides, and DNA [40]. Several factors induce biofilm formation including exposure to sublethal concentration of antibiotics.
In addition, biofilms can protect bacteria from antibiotics [48]. Therefore, the antivirulence potential of tested substances were evaluated by testing their ability to inhibit motility of E. coli treated with sub-MIC concentrations of ZnO NPs as illustrated in (Fig. 4), similar results were obtained with VC (data not shown). Crystal violet assay and electron microscope imaging were used to examine the antibiofilm activity against P. aeruginosa, cell clumps were formed, and biofilm was produced in untreated cells while in the presence of ZnO NPs/VC weekly adherent cells were formed that confirms the ability of the tested substances to inhibit biofilm maturation.
Conclusion
ZnO NPs, NaA, and vitamin C are safe alternatives to substitute conventional antibiotics. Our results revealed their promising antibacterial and antivirulent activity against the highly virulent E. coli O157:H7 and P. aeruginosa. Collectively, these results provide potential candidates to solve the problem of resistance to antibiotics. Eventually, we recommend more focused studies on the molecular mechanisms of their action and further in vivo evaluation for their safety using animal model.
Abbreviations
- AMR:
-
Antimicrobial resistance
- ATCC:
-
American Type Culture Collection
- CFU:
-
Colony forming unit
- NaA:
-
Sodium acetate
- MDR:
-
Multiple drug resistance
- MIC:
-
Minimum inhibitory concentration
- OD:
-
Optical density
- TEM:
-
Transmission electron microscope
- UV–Vis:
-
Ultraviolet- visible
- VC:
-
Vitamin C
- WHO:
-
World health organization.
- ZnO NPs:
-
Zinc oxide nanoparticles
References
Chahine EB et al (2022) Antibiotic approvals in the last decade: are we keeping up with resistance? Ann Pharmacother 56(4):441–462. https://doi.org/10.1177/10600280211031390
Roca I et al (2015) The global threat of antimicrobial resistance: science for intervention. New Microbes New Infect 6:22–29. https://doi.org/10.1016/j.nmni.2015.02.007
Lakoh S et al (2022) Incidence and risk factors of surgical site infections and related antibiotic resistance in Freetown, Sierra Leone: a prospective cohort study. Antimicrob Resist Infect Control 11(1):39. https://doi.org/10.1186/s13756-022-01078-y
Kelly MC et al (2021) Incidence and predictors of gram-negative bacilli in hospitalized people who inject drugs with injection drug use-attributable infections. Antimicrob Agents Chemother 65(12):e0092521. https://doi.org/10.1128/AAC.00925-21
Russell CD et al (2021) Co-infections, secondary infections, and antimicrobial use in patients hospitalised with COVID-19 during the first pandemic wave from the ISARIC WHO CCP-UK study: a multicentre, prospective cohort study. Lancet Microbe 2(8):e354–e365. https://doi.org/10.1016/S2666-5247(21)00090-2
Beyi AF et al (2017) Prevalence and antimicrobial susceptibility of Escherichia coli O157 in beef at butcher shops and restaurants in central Ethiopia. BMC Microbiol 17(1):49. https://doi.org/10.1186/s12866-017-0964-z
Guzzo F et al (2020) Plant derived natural products against Pseudomonas aeruginosa and staphylococcus aureus: antibiofilm activity and molecular mechanisms. Molecules. https://doi.org/10.3390/molecules25215024
D’Angelo F et al (2018) Identification of FDA-approved drugs as antivirulence agents targeting the pqs quorum-sensing system of Pseudomonas aeruginosa. Antimicrob Agents Chemother. https://doi.org/10.1128/AAC.01296-18
Zhao S et al (2020) Synthesis of Vitamin B12-antibiotic conjugates with greatly improved activity against gram-negative bacteria. Org Lett 22(16):6632–6636. https://doi.org/10.1021/acs.orglett.0c02403
Viault G et al (2021) Semisynthetic vitamin e derivatives as potent antibacterial agents against resistant gram-positive pathogens. ChemMedChem 16(5):881–890. https://doi.org/10.1002/cmdc.202000792
Gad El-Rab SM, Abo-Amer AE, Asiri AM (2020) Biogenic synthesis of ZnO nanoparticles and its potential use as antimicrobial agent against multidrug-resistant pathogens. Curr Microbiol 77(8):1767–1779
Eltaweil AS et al (2022) Novel biogenic synthesis of a Ag@ Biochar nanocomposite as an antimicrobial agent and photocatalyst for methylene blue degradation. ACS Omega 7(9):8046–8059
Hamed S et al (2015) N-acetyl cysteine substantially enhances the antimicrobial activity of zinc oxide nanoparticles against multidrug resistant pathogens. Int J Adv Res Biol Sci 2(12):1–15
Abdelfatah AM et al (2021) Green synthesis of nano-zero-valent iron using ricinus communis seeds extract: characterization and application in the treatment of methylene blue-polluted water. ACS Omega 6(39):25397–25411
Raghunath A, Perumal E (2017) Metal oxide nanoparticles as antimicrobial agents: a promise for the future. Int J Antimicrob Agents 49(2):137–152. https://doi.org/10.1016/j.ijantimicag.2016.11.011
Sánchez-López E et al (2020) Metal-Based nanoparticles as antimicrobial agents: an overview. Nanomaterials 10(2):292
Mumtaz S et al (2021) Evaluation of antibacterial activity of vitamin C against human bacterial pathogens. Braz J Biol 83:e247165. https://doi.org/10.1590/1519-6984.247165
Mousavi S, Bereswill S, Heimesaat MM (2019) Immunomodulatory and Antimicrobial Effects of Vitamin C. Eur J Microbiol Immunol (Bp) 9(3):73–79. https://doi.org/10.1556/1886.2019.00016
Doostan M et al (2021) Effective antibacterial electrospun cellulose acetate nanofibrous patches containing chitosan/erythromycin nanoparticles. Int J Biol Macromol 168:464–473. https://doi.org/10.1016/j.ijbiomac.2020.11.174
Tacconelli E et al (2018) Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis 18(3):318–327. https://doi.org/10.1016/S1473-3099(17)30753-3
Poole K (2001) Multidrug resistance in Gram-negative bacteria. Curr Opin Microbiol 4(5):500–508. https://doi.org/10.1016/s1369-5274(00)00242-3
Eichenberger EM, Thaden JT (2019) Epidemiology and mechanisms of resistance of extensively drug resistant gram-negative bacteria. Antibiotics (Basel). https://doi.org/10.3390/antibiotics8020037
Khalifa HO et al (2019) High Prevalence of antimicrobial resistance in gram-negative bacteria isolated from clinical settings in Egypt: recalling for judicious use of conventional antimicrobials in developing nations. Microb Drug Resist 25(3):371–385. https://doi.org/10.1089/mdr.2018.0380
Hamed S et al (2015) N-acetyl cysteine substantially enhances the antimicrobial activity of zinc oxidenanoparticles against multidrug resistant pathogens. Int J Adv Res Biol Sci 2(12):45–49
Nassar MSM, Hazzah WA, Bakr WMK (2019) Evaluation of antibiotic susceptibility test results: how guilty a laboratory could be? J Egypt Public Health Assoc 94(1):4. https://doi.org/10.1186/s42506-018-0006-1
CLSI (2015) Clinical and Laboratory Standards Institute 11 edition M07 (Methods for dilution antimicrobial susceptibility test for bacteria that grow aerobically). https://clsi.org/media/1928/m07ed11_sample.pdf
Koeth LM, DiFranco-Fisher Jeanna M, McCurdy.S, (2015) A reference Broth microdilution method for dalbavancin in vitro susceptibility testing of bacteria that grow aerobically. JoVE. https://doi.org/10.3791/53028
Zhang Y et al (2019) Synergistic Effect of colistin combined with PFK-158 against colistin-Resistant Enterobacteriaceae. Antimicrob Agents Chemother. https://doi.org/10.1128/AAC.00271-19
Odds FC (2003) Synergy, antagonism, and what the chequerboard puts between them. J Antimicrob Chemother 52(1):1. https://doi.org/10.1093/jac/dkg301
Bhattacharya D et al (2020) Anti-virulence activity of polyphenolic fraction isolated from kombucha against Vibrio cholerae. Microb Pathog 140:103927. https://doi.org/10.1016/j.micpath.2019.103927
Samarasinghe S, Reid R, Al-Bayati M (2019) The anti-virulence effect of cranberry active compound proanthocyanins (PACs) on expression of genes in the third-generation cephalosporin-resistant Escherichia coli CTX-M-15 associated with urinary tract infection. Antimicrob Resist Infect Control 8:181. https://doi.org/10.1186/s13756-019-0637-9
Ha DG, Kuchma SL, O’Toole GA (2014) Plate-based assay for swimming motility in Pseudomonas aeruginosa. Methods Mol Biol 1149:59–65. https://doi.org/10.1007/978-1-4939-0473-0_7
Chen P et al (2019) Size-dependent cytotoxicity study of ZnO nanoparticles in HepG2 cells. Ecotoxicol Environ Saf 171:337–346. https://doi.org/10.1016/j.ecoenv.2018.12.096
Elsayed EA, Sharaf-Eldin MA, Wadaan M (2015) In vitro evaluation of cytotoxic activities of essential oil from moringa oleifera seeds on HeLa, HepG2, MCF-7, CACO-2 and L929 Cell Lines. Asian Pac J Cancer Prev 16(11):4671–4675. https://doi.org/10.7314/apjcp.2015.16.11.4671
Wu H et al (2022) Changes of antibiotic resistance over time among Escherichia coli peritonitis in Southern China. Perit Dial Int 42(2):218–222. https://doi.org/10.1177/08968608211045272
Kubes JN, Fridkin SK (2019) Factors affecting the geographic variability of antibiotic-resistant healthcare-associated infections in the United States using the CDC antibiotic resistance patient safety Atlas. Infect Control Hosp Epidemiol 40(5):597–599. https://doi.org/10.1017/ice.2019.64
Hamed.S, et al (2017) N-acetylcysteine potentiates the antimicrobial activity of gold nanoparticles a paradigm shift in treatment of multidrug resistant pathogens. EC Microbiology 10(3):12
Cabezas-Pizarro J et al (2018) Antimicrobial activity of different sodium and potassium salts of carboxylic acid against some common foodborne pathogens and spoilage-associated bacteria. Rev Argent Microbiol 50(1):56–61. https://doi.org/10.1016/j.ram.2016.11.011
Hurtukova K et al (2021) Antibacterial properties of a honeycomb-like pattern with cellulose acetate and silver nanoparticles. Materials (Basel). https://doi.org/10.3390/ma14144051
Xu C et al (2022) Bactericidal anti-biofilm and anti-virulence activity of vitamin C against carbapenem-resistant hypervirulent Klebsiella pneumoniae. iScience. 25(3):103894. https://doi.org/10.1016/j.isci.2022.103894
Leber AL (2016) Synergy testing: broth microdilution checkerboard and broth macrodilution methods. In: Eisenberg IHD (ed) Clinical microbiology procedures handbook, vol 24. American Society for Microbiology press, Washington
Ogese MO et al (2017) Characterization of drug-specific signaling between primary human hepatocytes and immune cells. Toxicol Sci 158(1):76–89. https://doi.org/10.1093/toxsci/kfx069
Nicolay W et al (2021) Characterization of RNA Sensing Pathways in Hepatoma Cell Lines and Primary Human Hepatocytes. Cells. https://doi.org/10.3390/cells10113019
Hamed S et al (2019) Synergistic action of SPI-1 gene expression in Salmonella enterica serovar typhimurium through transcriptional crosstalk with the flagellar system. BMC Microbiol 19(1):211. https://doi.org/10.1186/s12866-019-1583-7
Huang Y et al (2008) Formate acts as a diffusible signal to induce Salmonella invasion. J Bacteriol 190(12):4233–4241. https://doi.org/10.1128/JB.00205-08
Golubeva YA et al (2016) Intestinal long-chain fatty acids act as a direct signal to modulate expression of the salmonella pathogenicity Island 1 Type III secretion system. mbio 7(1):e02170-15. https://doi.org/10.1128/mBio.02170-15
Hamed S et al (2021) HilE is required for synergistic activation of SPI-1 gene expression in Salmonella enterica serovar typhimurium. BMC Microbiol 21(1):49. https://doi.org/10.1186/s12866-021-02110-8
Martinez M et al (1861) (2019) Synergistic and antibiofilm activity of the antimicrobial peptide P5 against carbapenem-resistant Pseudomonas aeruginosa. Biochim Biophys Acta Biomembr 7:1329–1337. https://doi.org/10.1016/j.bbamem.2019.05.008
Acknowledgements
Not applicable.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). No grants were received during this research work, but we would like to thank Faculty of pharmacy, Helwan University, Egypt, for using its resources.
Author information
Authors and Affiliations
Contributions
SH conceived the idea, designed the research, and acquired and analysed the data; SH and ME wrote the manuscript. All authors have read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Authors declare that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hamed, S., Emara, M. Antibacterial and Antivirulence Activities of Acetate, Zinc Oxide Nanoparticles, and Vitamin C Against E. coli O157:H7 and P. aeruginosa. Curr Microbiol 80, 57 (2023). https://doi.org/10.1007/s00284-022-03151-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00284-022-03151-6