Abstract
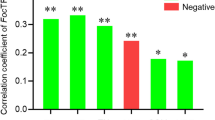
Fusarium wilt caused by Fusarium oxysporum f. sp. niveum is an important manifestation of continuous cropping barrier, which causes the quality and yield of watermelon to decline. In early stage of this study, the organic fertilizer fermented by Bama pig manure applied in soil was proved to significantly inhibit the occurrence of disease by improving the structure of soil microbial community. However, the mechanism was not clear. The high-throughput sequencing technology, combined with network and PICRUSt2 function analysis was used to investigate it. MiSeq sequencing showed that the bacterial community of organic fertilizer treated soil was composed of 34 phyla and 768 genera, the number of genera was higher than that of sterile water treated soil. Fertilization significantly increased the diversity and changed the composition of bacterial community based on alpha, beta diversity, and ANOSIM/Adonis analysis. LEfSe species difference and network analysis showed that fertilization improved the relative abundance of bacteria with biological control or plant growth promotion characteristics in soil, such as Sphingomonas, Halobacillus, Nocardioides, and enhanced the interaction between rhizosphere bacteria, made the network structure more complex. PICRUSt2 also revealed fertilization promoted the bacterial function, such as metabolism and genetic information processing. These results showed that the pig manure organic fertilizer might reduce the occurrence of Fusarium wilt by regulating bacterial community, interaction, and functional metabolism in watermelon rhizosphere soil.





Similar content being viewed by others
Data Availability
The Illumina MiSeq sequence data were deposited in the Sequence Read Archive (SRA) of the National Center of Biotechnology Information (NCBI) database with the accession number PRJNA814193.
Code Availability
Not applicable.
References
Wei X, Zhao Y, Yang R (2016) Advances on watermelon continuous cropping obstacles and its prevention measures under protective cultivation. China Cucurbits Vege 29(9):1–5. https://doi.org/10.16861/j.cnki.zggc.2016.0162
Zhang X, Huo Z, Wu Z, Cheng Z (2015) Analysis on the causes of continuous cropping barrier of watermelon and its control strategies. Agr Sci Technol 16(3):180–185. https://doi.org/10.16175/j.cnki.1009-4229.2015.03.012
Zhang H, Hua ZW, Liang WZ, Niu QH, Wang X (2020) The prevention of bio-organic fertilizer fermented from cow manure compost by Bacillus sp. XG-1 on watermelon continuous cropping barrier. Int J Environ Res Public Health 17(16):5714. https://doi.org/10.3390/ijerph17165714
Wang K (2019) Analysis of obstacles and overcoming measures of continuous watermelon cropping. China Fruit Veg 39(03):70–72. https://doi.org/10.19590/j.cnki.1008-1038.2019.03.015
Faheem M, Raza W, Zhong W, Nan Z, Xu Y (2014) Evaluation of the biocontrol potential of Streptomyces goshikiensis YCXU against Fusarium oxysporum f. sp. niveum. Biol Control 81:101–110. https://doi.org/10.1016/j.biocontrol.2014.11.012
Dickinson M (2012) Plant fungal pathogens: methods and protocols. Plant Pathol 61(6):1187–1187. https://doi.org/10.1111/j.1365-3059.2012.02633.x
Wang B, Yuan J, Zhang J, Shen Z, Zhang M, Li R, Ruan Y, Shen Q (2013) Effects of novel bioorganic fertilizer produced by Bacillus amyloliquefaciens W19 on antagonism of Fusarium wilt of banana. Biol Fertil Soils 49(4):435–446. https://doi.org/10.1007/s00374-012-0739-5
Zhang N, Yang D, Wang D, Miao Y, Shao J, Zhou X, Xu Z, Li Q, Feng H, Li S, Shen Q, Zhang R (2015) Whole transcriptomic analysis of the plant-beneficial rhizobacterium Bacillus amyloliquefaciens SQR9 during enhanced biofilm formation regulated by maize root exudates. BMC Genom 16(1):685. https://doi.org/10.1186/s12864-015-1825-5
Xie S, Jiang H, Ding T, Xu Q, Chai W, Cheng B (2018) Bacillus amyloliquefaciens FZB42 represses plant miR846 to induce systemic resistance via a jasmonic acid-dependent signalling pathway. Mol Plant Pathol 19(7):1612–1623. https://doi.org/10.1111/mpp.12634
Ling N, Raza W, Ma J, Huang Q, Shen Q (2011) Identification and role of organic acids in watermelon root exudates for recruiting Paenibacillus polymyxa SQR-21 in the rhizosphere. Eur J Soil Biol 47(6):374–379. https://doi.org/10.1016/j.ejsobi.2011.08.009
Arseneault T, Goyer C, Filion M (2013) Phenazine production by Pseudomonas sp. LBUM223 contributes to the biological control of potato common scab. Phytopathology 103(10):995–1000. https://doi.org/10.1094/PHYTO-01-13-0022-R
Law JW, Ser H, Khan TM, Chuah L, Pusparajah P, Chan K, Goh B, Lee L (2017) The potential of Streptomyces as biocontrol agents against the rice blast Fungus, Magnaporthe oryzae (Pyricularia oryzae). Front Microbiol 8:3. https://doi.org/10.3389/fmicb.2017.00003
Liu F, Tian W, Li LZ, Yang XM, Shen B, Shen QR (2013) Optimization of solid-state fermentation conditions for antagonistic Bacillus subtilis SQR9 producing bio-organic fertilizer. Chin J Appl Environ Biol 19(1):90–95. https://doi.org/10.3724/SP.J.1145.2013.00090
Aldahmani JH, Abbasi PA, Sahin F, Hoitink HAJ, Miller SA (2005) Reduction of bacterial leaf spot severity on radish, lettuce, and tomato plants grown in compost-amended potting mixes. Can J Plant Pathol 27(2):186–193. https://doi.org/10.1080/07060660509507215
Millner P (2006) Control of strawberry black root rot with compost socks. Plant Health Prog 10:1–5. https://doi.org/10.1094/PHP-2006-1016-02-RS
Horst LE, Locke J, Krause CR, McMahon RW, Madden LV, Hoitink HAJ (2005) Suppression of botrytis blight of begonia by Trichoderma hamatum 382 in peat and compost-amended potting mixes. Plant Dis 89(11):1195–1200. https://doi.org/10.1094/PD-89-1195
Huang J, Li H, Yuan H (2006) Effect of organic amendments on Verticillium wilt of cotton. Crop Prot 25(11):1167–1173. https://doi.org/10.1016/j.cropro.2006.02.014
Wood JL, Tang CX, Franks AE (2016) Microbial associated plant growth and heavy metal accumulation to improve phytoextraction of contaminated soils. Soil Biol Biochem 103:131–137. https://doi.org/10.1016/j.soilbio.2016.08.021
He Y, Zhang Y, Zhu FY, Xiao JL, Wei L, Tang YY, Liang ZH (2020) Analysis of rhizosphere microbial community structure in different pathogenesis stages of watermelon Fusarium Wilt. Agric Sci Technol 21(05):19–25. https://doi.org/10.16175/j.cnki.1009-4229.2020.02.004
Zhu FY, Li JG, Zhang Y, Xiao JL, Liang ZH (2018) Watermelon rhizosphere soil bacterial diversity affects the occurrence of Fusarium Wilt. Chin Agric Sci Bull 34(17):69–76
Tang LL, Xia Y, Fan C, Kou JM, Wu FZ, Li WH, Pan K (2020) Control of Fusarium wilt by wheat straw is associated with microbial network changes in watermelon rhizosphere. Sci Rep 10(1):12736. https://doi.org/10.1038/s41598-020-69623-6
Chen ZJ, Zheng Y, Ding CY, Ren XM, Yuan J, Sun F, Li YY (2017) Integrated metagenomics and molecular ecological network analysis of bacterial community composition during the phytoremediation of cadmium-contaminated soils by bioenergy crops. Ecotoxicol Environ Saf 145:111–118. https://doi.org/10.1016/j.ecoenv.2017.07.019
Chen ZJ, Xu G, Ding CY, Zheng BH, Chen Y, Han H, Li YY, Shi JW, Hu LQ (2020) Illumina MiSeq sequencing and network analysis the distribution and co-occurrence of bacterioplankton in Danjiangkou Reservoir, China. Arch Microbiol 202(4):859–873. https://doi.org/10.1007/s00203-019-01798-7
Wang M, Chen SB, Chen L, Wang D (2019) Responses of soil microbial communities and their network interactions to saline-alkaline stress in Cd-contaminated soils. Environ Pollut 252(Pt B):1609–1621. https://doi.org/10.1016/j.envpol.2019.06.082
Li B, Li YS, Wei JB, Song XY, Shi RJ, Hou YX, Liu SY (2020) Effects of different land use typess on the molecular ecological network of soil bacteria. Environ Sci 41(03):1456–1465. https://doi.org/10.13227/j.hjkx.201907179
Chen ZJ, Lin LA, Li YJ, Chen Y, Zhang H, Han H, Wu NC, Nicola F, Li YY, Ren XM (2021) Shifts in rhizosphere bacterial community structure, co-occurrence network, and function of Miscanthus following cadmium exposure. Environ Sci 42(08):3997–4006. https://doi.org/10.13227/j.hjkx.202011198
Bulgarelli D, Rott M, Schlaeppi K, Themaat EVL, Ahmadinejad N, Assenza F, Rauf P, Huettel B, Reinhardt R, Schmelzer E, Peplies J, Gloeckner FO, Amann R, Eickhorst T, Schulze-Lefert P (2012) Revealing structure and assembly cues for Arabidopsis root-inhabiting bacterial microbiota. Nature 488(7409):91–95. https://doi.org/10.1038/nature11336
Zhang ZG, Zhang JY, Wang YC, Zheng XB (2005) Molecular detection of Fusarium oxysporum f. sp. niveum and Mycosphaerella melonis in infected plant tissues and soil. FEMS Microbiol Lett 249(1):39–47. https://doi.org/10.1016/j.femsle.2005.05.057
Segata N, Lzard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, Huttenhower C (2011) Metagenomic biomarker discovery and explanation. Genome Biol 12(6):R60. https://doi.org/10.1186/gb-2011-12-6-r60
Khan AL, Waqas M, Asaf S, Kamran M, Shahzad R, Bilal S, Khan MA, Kang SM, Kim YH, Yun BW, Al-Rawahi A, Al-Harrasi A, Lee IJ (2017) Plant growth-promoting endophyte Sphingomonas sp. LK11 alleviates salinity stress in Solanum pimpinellifolium. Environ Exp Bot 133:58–69. https://doi.org/10.1016/j.envexpbot.2016.09.009
Yang L, Tan RX, Wang Q, Huang WY, Yin YX (2002) Antifungal cyclopeptides from Halobacillus litoralis YS3106 of marine origin. Tetrahedron Lett 43(37):6545–6548. https://doi.org/10.1016/S0040-4039(02)01458-2
Alenezi FN (2020) Biological Control potential of Aneurinibacillus migulanus against Phytophthora species. Fungal Genom Biol 10(2):1–4. https://doi.org/10.35248/2165-8056.20.10.165
Morimura H, Ito M, Yoshida S, Koitabashi M, Tsushima S, Camagna M, Chiba S, Takemoto D, Kawakita K, Sato I (2020) In vitro assessment of biocontrol effects on Fusarium head blight and deoxynivalenol (DON) accumulation by DON-degrading bacteria. Toxins 12(6):399. https://doi.org/10.3390/toxins12060399
Pérez-Jaramillo JE, Hollander MD, Ramírez CA, Mendes R, Raaijmakers JM, Carrión VJ (2019) Deciphering rhizosphere microbiome assembly of wild and modern common bean (Phaseolus vulgaris) in native and agricultural soils from Colombia. Microbiome 7(1):114. https://doi.org/10.1186/s40168-019-0727-1
Chen DL, Wang XX, Zhang W, Zhou ZG, Ding CF, Liao YWK, Li XG (2020) Persistent organic fertilization reinforces soil-borne disease suppressiveness of rhizosphere bacterial community. Plant Soil 452:313–328. https://doi.org/10.1007/s11104-020-04576-3
Wu ZX, Liu QL, Li HH (2021) PICRUSt analysis of the effect of organic fertilizer on the functional composition of bacterial community in degraded red soil. Jiangsu Agric Sci 49(16):60–66. https://doi.org/10.15889/j.issn.1002-1302.2021.16.010
Coyte KZ, Schluter J, Foster KR (2015) The ecology of the microbiome: networks, competition, and stability. Science 350(6261):663–666. https://doi.org/10.1126/science.aad2602
Layeghifard M, Hwang DM, Guttman DS (2017) Disentangling interactions in the microbiome: a network perspective. Trends Microbiol 25(3):217–228. https://doi.org/10.1016/j.tim.2016.11.008
Wang XH, Ma Q, Tian Y, Hu J, Liu HR (2019) Cultivable Myxobacteria and their antibiotic activities in the Hulun Buir Area of Inner Mongolia. Biotechnol Bull 35(09):224–233. https://doi.org/10.13560/j.cnki.biotech.bull.1985.2019-0210
Raza W, Faheem M, Yousaf S, Rajer F, Yamin M (2013) Volatile and non-volatile antifungal compounds produced by Trichoderma harzianum SQR-T037 suppressed the growth of Fusarium oxysporum f. sp. niveum. Sci Lett 1(1):21–24
Chen X, Koumoutsi A, Scholz R, Eisenreich A, Schneider K, Heinemeyer I, Morgenstern B, Voss B, Hess W, Reva O (2007) Comparative analysis of the complete genome sequence of the plant growth-promoting bacterium Bacillus amyloliquefaciens FZB42. Nat Biotechnol 25(9):1007–1014. https://doi.org/10.1038/nbt1325
Liu JA, Shu AP, Song WF, Shi WC, Li MC, Zhang WX, Li ZZ, Liu GR, Yuan FS, Zhang SX, Liu ZB, Gao Z (2021) Long-term organic fertilizer substitution increases rice yield by improving soil properties and regulating soil bacteria. Geoderma 404:115287. https://doi.org/10.1016/J.GEODERMA.2021.115287
Raza W, Yuan J, Ling N, Huang Q, Shen QR (2015) Production of volatile organic compounds by an antagonistic strain Paenibacillus polymyxa WR-2 in the presence of root exudates and organic fertilizer and their antifungal activity against Fusarium oxysporum f. sp. niveum. Biol Control 80:89–95. https://doi.org/10.1016/j.biocontrol.2014.09.004
Xu W, Wang K, Wang H, Liu Z (2020) Evaluation of the biocontrol potential of Bacillus sp. WB against Fusarium oxysporum f. sp. niveum. Biol Control 147:104288. https://doi.org/10.1016/j.biocontrol.2020.104288
Ning L, Huang Q, Guo S, Shen QR (2011) Paenibacillus polymyxa SQR-21 systemically affects root exudates of watermelon to decrease the conidial germination of Fusarium oxysporum f. sp. niveum. Plant Soil 341(1/2):485–493. https://doi.org/10.1007/s11104-010-0660-3
Xu Z, Shao J, Li B, Yan X, Shen QR, Zhang RF (2013) Contribution of Bacillomycin D in Bacillus amyloliquefaciens SQR9 to antifungal activity and biofilm formation. Appl Environ Microbiol 79(3):808–815. https://doi.org/10.1128/AEM.02645-12
Guo L, Li C, Liu PS, Chang JF, Zhou XT, Zhang N, Zhu ZY, Weng YW, Ma YH (2021) Effect of replacing chemical fertilizer with cow manure organic fertilizer on tea yield, quality, and soil fertility in tea garden. J Soil Water Conserv 35(06):264–269. https://doi.org/10.13870/j.cnki.stbcxb.2021.06.036
Acknowledgements
The authors would like to acknowledge Dr. Xiang Wang for sharing the idea of this work and Proof-Reading-Service for language editing.
Funding
This work was supported by the Natural Science Foundation of Henan Province (212300410215), the National Natural Science Fund of China (U2004145), the key research and development projects of Henan Province (221111520600), the higher discipline innovation and talent introduction base of Henan Province (No. CXJD2019001) and the High-qualified Talents Scientific Research Startup Foundation of Nanyang Normal University (2019ZX015).
Author information
Authors and Affiliations
Contributions
HZ and ZJC designed the research. HZ, XZ, JS, and HYY conducted the experiments. LZD collected soil samples. HH provided advices during the study. HZ and XZ drafted the manuscript. ZJC reviewed the manuscript. HYY and ZJC revised the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors report no conflicts of interest.
Ethical Approval
Not applicable.
Consent to Participate
Not applicable.
Consent to Publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, H., Yang, H., Zhang, X. et al. Bama Pig Manure Organic Fertilizer Regulates the Watermelon Rhizosphere Bacterial Community to Inhibit the Occurrence of Fusarium Wilt Under Continuous Cropping Conditions. Curr Microbiol 79, 364 (2022). https://doi.org/10.1007/s00284-022-03056-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00284-022-03056-4