Abstract
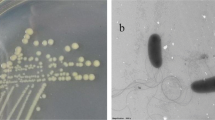
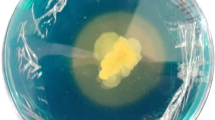
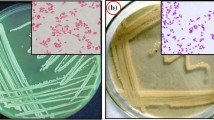
An efficient siderophore producing strain, YQ9, was isolated from heavy metal contaminated soil and identified as Burkholderia vietnamiensis. To the best of our known, the strain owns the highest siderophore producing capacity among genus Burkholderia with 96.6% siderophore unit. Moreover, B. vietnamiensis YQ9 has good adaptability to different pH values, temperatures, NaCl, and Fe3+ concentrations. In addition, the minimum inhibitory concentration (MIC) of heavy metals and antibiotics were also tested. It was found that the MIC values of strain YQ9 to several major soil heavy metal pollutants, such as Pb2+, Zn2+, Cu2+, and Cd2+ reached 3000, 5000, 4500, and 1000 μmol·L−1, respectively. And YQ9 was sensitive to 4 of 8 test antibiotics, including rifampicin, kanamycin, doxycycline hyclate, and gentamicin (25, 25, 30, and 30 μg·mL−1, respectively). Strain YQ9 also owns the ability to produce indole-3-acetic acid (IAA) and 1-aminocyclopropane-1-carboxylic acid (ACC) deaminase and dissolve phosphorus. The IAA production capacity was 6.93 mg·L−1, the ACC deaminase activity was 8.71 μmol α-KA·(h·mg)−1, and the phosphorus dissolving capacity of YQ9 was 104.05 mg·L−1. The traits were excellent, and the strain was qualified as a candidate for microbial reinforcement of phytoremediation in soil contaminated by heavy metals.




Similar content being viewed by others
Data Availability
The dataset generated during and/or analyzed within the current study are available from the corresponding author on reasonable request.
References
Li YQ, Ma JW, Shafi M, Sun YF, Li YQ, Chen ZH, Xiang ZC, Jin GQ, Zhong B, Ye ZQ, Liu D (2020) Effects of adsorption characteristics of different amendments on heavy metals (Pb, Zn, and Cd). J Soil Sediment 20:2868–2876. https://doi.org/10.1007/s11368-020-02620-4
Guo JK, Dong MF, Ding YZ, Feng RW, Wang RG, Xu YM (2015) Effects of plant growth promoting rhizobacteria on plants heavy metal uptake and transport: a review. Ecol Environ Sci 24:1228–1234. https://doi.org/10.16258/j.cnki.1674-5906.2015.07.023
Sana A, Qasim A, Zahir AZ, Sobia A, Hafiz NA (2019) Phytoremediation: environmentally sustainable way for reclamation of heavy metal polluted soils. Ecotox Environ Safe 174:714–727. https://doi.org/10.1016/j.ecoenv.2019.02.068
McGrath SP, Zhao J, Lombi E (2002) Phytoremediation of metals, metalloids, and radionuclides. Adv Agron 75:1–56. https://doi.org/10.1016/s0065-2113(02)75002-5
Ashraf S, Ali Q, Zahir ZA, Ashraf S, Asghar HN (2019) Phytoremediation: environmentally sustainable way for reclamation of heavy metal polluted soils. Ecotoxicol Environ Saf 174:714–727. https://doi.org/10.1016/j.ecoenv.2019.02.068
Naeem K, Asghari B (2016) Modulation of phytoremediation and plant growth by the treatment with PGPR, Ag nanoparticle and untreated municipal wastewater. Int J Phytoremediat 18:1258–1269. https://doi.org/10.1080/15226514.2016.1203287
Sinha S, Mukherjee SK (2008) Cadmium-induced siderophore production by a high Cd-resistant bacterial strain relieved Cd toxicity in plants through root colonization. Curr Microbiol 56:55–60. https://doi.org/10.1007/s00284-007-9038-z
Sinha AK, Parli VB, Tripathy SC, Sarkar A, Prabhakaran S (2019) Effects of growth conditions on siderophore producing bacteria and siderophore production from Indian Ocean sector of Southern Ocean. J Basic Microbiol 59(4):412–424. https://doi.org/10.1002/jobm.201800537
Pedersen K, Lundstrom US, Bylund D, Essen SA, Johnsson A (2007) Siderophore production by Pseudomonas stutzeri under aerobic and anaerobic conditions. Appl Environ Microb 73:5857–5864. https://doi.org/10.1128/aem.00072-07
Saha M, Sarkar S, Sarkar B, Sharma BK, Bhattacharjee S, Tribedi P (2016) Microbial siderophores and their potential applications: a review. Environ Sci Pollut Res 23(5):3984–3999. https://doi.org/10.1007/s11356-015-4294-0
Upreti R, Thomas P (2015) Root-associated bacterial endophytes from Ralstonia solanacearum resistant and susceptible tomato cultivars and their pathogen antagonistic effects. Front Microbiol 6(255):255. https://doi.org/10.3389/fmicb.2015.00255
Skaar EP (2010) The battle for iron between bacterial pathogens and their vertebrate hosts. PLoS Pathog 6:e1000949. https://doi.org/10.1371/journal.ppat.1000949
Zhang L (2014) Bioaugmentation with siderophore-producing bacteria to enhance phytoremediation of heavy metal polluted soil by sweet sorghum. Environ Sci Technol 37:74–79. https://doi.org/10.3969/j.issn.1003-6504.2014.04.015
Braud A, Jezequel K, Bazot S, Lebeau T (2009) Enhanced phytoextraction of an agricultural Cr- and Pb-contaminated soil by bioaugmentation with siderophore-producing bacteria. Chemosphere 74:280–286. https://doi.org/10.1016/j.chemosphere.2008.09.013
Tripathi M, Munot HP, Shouche Y, Meyer JM, Goel R (2005) Isolation and functional characterization of siderophore-producing lead- and cadmium-resistant Pseudomonas putida KNP9. Curr Microbiol 50:233–237. https://doi.org/10.1007/s00284-004-4459-4
Bao S (2000) Agronomic analysis of soils. China Agricultural Machinery Press, Beijing
Mani R, Helena F (2008) Effects of inoculation of plant-growth promoting bacteria on Ni uptake by Indian mustard. Bioresour Technol 99:3491–3498. https://doi.org/10.1016/j.biortech.2007.07.046
Wang WX, Zhou XL, Zhong-Ling LI, Wang MP, Wang WW (2014) Detection of siderophore production from hydrogen-oxidizing bacteria with CAS overlay plate method. Microb China 41:1692–1697. https://doi.org/10.13344/j.microbiol.china.130462
Arora NK, Verma M (2017) Modified microplate method for rapid and efficient estimation of siderophore produced by bacteria. 3 Biotech 7:381. https://doi.org/10.1007/s13205-017-1008-y
Holt JG, Krieg NR, Sneath PHA, Staley JT, Williams ST (1994) Bergey’s manual of determinative bacteriology, 9th edn. Williams & Wilkins, Baltimore
Arjomandzadegan M, Sadrnia M, Titov L, Surkova L, Sarmadian H, Ghasemikhah R, Hosseiny H (2017) Transmission electron microscopy of XDR mycobacterium tuberculosis isolates grown on high dose of ofloxacin. Sci Pharm 85(1):1–7. https://doi.org/10.3390/scipharm85010003
Arnow LE (1937) Colorimetric determination of the components of 3, 4-dihydroxyphenylalanine tyrosine mixtures. J Biol Chem 118(2):531–537. https://doi.org/10.1111/j.1469-185X.1937.tb01231.x
Csaky TZ, Hassel O, Rosenberg T, Lang S, Turunen E, Tuhkanen A (1948) On the estimation of bound hydroxylamine in biological materials. Acta Chem Scand 2:450–454. https://doi.org/10.3891/acta.chem.scand.02-0450
Shenker M, Oliver I, Helmann M, Hadar Y, Chen Y (1992) Utilization by tomatoes of iron mediated by a siderophore produced by Rhizopus arrhizus. J Plant Nutr 15:2173–2182. https://doi.org/10.1080/01904169209364466
Filali BK, Taoufik J, Zeroual Y, Dzairi FZ, Talbi M, Blaghen M (2000) Waste water bacterial isolates resistant to heavy metals and antibiotics. Curr Microbiol 41:151–156. https://doi.org/10.1007/s0028400
Li ZD, Chen XR, Li P, Man BY (2010) Identification of Polygonum viviparum endophytic bacteria Z5 and determination of the capacity to secrete IAA and antagonistic capacity towards pathogenic fungi. Acta Pratacult Sci 19:61–68. https://doi.org/10.11686/cyxb20100209
Nautiyal CS (1999) An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Microbiol Lett 170:265–270. https://doi.org/10.1111/j.1574-6968.1999.tb13383.x
Cao X, Wang X, Dong Z, Shen Q, Deng N, Li Y (2018) Isolation and identification of potassium dissolving bacteria in Karst soil. Genomics Appl Biol 4:1487–1494. https://doi.org/10.13417/j.gab.037.001487
Chen Q, Hu HY, Gao M, Xu J, Zhou YQ, Sun JG (2011) Screening and identification of a nitrogen fixing bacteria with 1-aminocyclopropane-1-carboxylate deaminase activity. Plant Nutr Fertil Sci 17:1515–1521
GB 15618-2018. 2018. Soil Environmental Quality Risk Control Standard for Soil Contamination of Agricultural Land. Ministry of Ecology and Environment of China, and State Administration for Market Regulation of China. p. 4.
Van VT, Berge O, Ke SN, Balandreau J, Heulin T (2000) Repeated beneficial effects of rice inoculation with a strain of Burkholderia vietnamiensis on early and late yield components in low fertility sulphate acid soils of Vietnam. Plant Soil 218:273–284. https://doi.org/10.1023/A:1014986916913
Li P, Liu D, Zhu XL, Jiang XB, Bi ZY (2017) Screening, identification and inhibitory effect of antagonistic bacterium on Fusarium oxysporum f. sp. Nevium. J ZK Univ Agric Eng 30:24–28. https://doi.org/10.3969/j.issn.1674-5663.2017.03.005
Lin TX, Tang M, Huang MY, Guan QL, Gong MF (2012) Screening and identification of a high yield siderophores-producing bacteria SS05 isolated from cotton soil. Microbiol China 39:668–676. https://doi.org/10.13344/j.microbiol.china.2012.05.005
Singh TB, Sahai V, Goyal D, Prasad M, Yadav A, Shrivastav P, Ali A, Dantu PK (2020) Identification, characterization and evaluation of multifaceted traits of plant growth promoting rhizobacteria from soil for sustainable approach to agriculture. Curr Microbiol 77:3633–3642. https://doi.org/10.1007/s00284-020-02165-2
Gonin M, Gensous S, Lagrange A, Ducousso M, Amir H, Jourand P (2013) Rhizosphere bacteria of Costularia spp. from ultramafic soils in new Caledonia: diversity, tolerance to extreme edaphic conditions, and role in plant growth and mineral nutrition. Can J Microbiol 59:164–174. https://doi.org/10.1139/cjm-2012-0570
Sayyed RZ, Seifi S, Patel PR, Shaikh SS, Enshasy HE (2019) Siderophore production in groundnut rhizosphere isolate, Achromobacter sp. RZS2 influenced by physicochemical factors and metal ions. Environ Sustain 2:117–124. https://doi.org/10.1007/s42398-019-00070-4
Jiang J, Pan CH, Xiao AP, Yang XA, Zhang GM (2017) Isolation, identification, and environmental adaptability of heavy-metal-resistant bacteria from ramie rhizosphere soil around mine refinery. 3 Biotech 7:1–6. https://doi.org/10.1007/s13205-017-0603-2
Sridevi M, Mallaiah KV (2008) Production of hydroxamate-type of siderophores by Rhizobium strains from Sesbania sesban (L.) Merr. Int J Soil Sci 3:28–34. https://doi.org/10.3923/ijss.2008.28.34
Dimkpa CO, Svatos A, Dabrowska P, Schmidt A, Boland W, Kothe E (2008) Involvement of siderophores in the reduction of metal-induced inhibition of auxin synthesis in Streptomyces spp. Chemosphere 74:19–25. https://doi.org/10.1016/j.chemosphere.2008.09.079
Guo JK, Tang SR, Ju XH, Ding YZ, Liao SQ, Song NN (2011) Effects of inoculation of a plant growth promoting rhizobacterium Burkholderia sp. D54 on plant growth and metal uptake by a hyperaccumulator Sedum alfredii Hance grown on multiple metal contaminated soil. World J Microb Biotechnol 27:2835–2844. https://doi.org/10.1007/s11274-011-0762-y
Liu X, Hu X, Cao Y, Pang W, Huang J, Guo P, Huang L (2019) Biodegradation of phenanthrene and heavy metal removal by acid-tolerant Burkholderia fungorum FM-2. Front Microbiol. https://doi.org/10.3389/fmicb.2019.0040
Ka-Ot AL, Banerjee S, Haldar G, Joshi SR (2017) Acid and heavy metal tolerant Bacillus sp. from rat-hole coal mines of Meghalaya. India. Proc Natl Acad Sci India 88:1–12. https://doi.org/10.1007/s40011-017-0856-x
Yu FB, Shen B, Li SP (2006) Isolation and characterization of Pseudomonas sp. strain ONBA-17 degrading o-nitriobenzaldehyde. Curr Microbiol 53:457–461. https://doi.org/10.1007/s00284-005-0380-8
Sütterlin S, Téllez-Castillo CJ, Anselem L, Yin H, Bray JE, Maiden MCJ (2018) Heavy metal susceptibility on Escherichia coli from urine samples from Sweden, Germany and Spain. Antimicrob Agents Chemother. https://doi.org/10.1128/AAC.00209-18
Tiwari S, Sarangi BK, Thul ST (2016) Identification of arsenic resistant endophytic bacteria from Pteris vittata roots and characterization for arsenic remediation application. J Environ Manage 180:359–365. https://doi.org/10.1016/j.jenvman.2016.05.029
Khan MS, Zaidi A, Wani PA, Oves M (2010) Role of plant growth promoting rhizobacteria in the remediation of metal contaminated soils: a review. Environ Chem Lett 7:1–19. https://doi.org/10.1007/s10311-008-0155-0
Manzoor M, Abbasi MK, Sultan T (2016) Isolation of phosphate solubilizing bacteria from maize rhizosphere and their potential for rock phosphate solubilization–mineralization and plant growth promotion. Geomicrobiol J 34:81–95. https://doi.org/10.1088/1755-1315/439/1/012
Tagele SB, Kim SW, Lee HG, Lee YS (2019) Potential of novel sequence type of Burkholderia cenocepacia for biological control of root rot of maize (Zea mays L.) caused by Fusarium temperatum. Int J Mol Sci 20:1005. https://doi.org/10.3390/ijms20051005
Jiang CY, Sheng XF, Qian M, Wang QY (2008) Isolation and characterization of a heavy metal-resistant Burkholderia sp. from heavy metal-contaminated paddy field soil and its potential in promoting plant growth and heavy metal accumulation in metal-polluted soil. Chemosphere 72:157–164. https://doi.org/10.1016/j.chemosphere.2008.02.006
Funding
This work was supported by the grant from Key Innovation Team Project in Zhejiang province (2013TD12).
Author information
Authors and Affiliations
Contributions
Writing—Original Draft: YW. Visualization: YW and YL. Formal analysis: YW, WH, and YL. Resources, Writing—Review & Editing, Supervision: FBY, SWA and HHD.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research Involving Human and Animal Participants
This research does not involve studies with human participants or animals.
Consent for Publication
All authors read and approved the final manuscript. The work described has not been published. It is not under consideration for publication elsewhere. Its publication has been approved by all coauthors. If the manuscript is accepted for publication, the authors agree to automatic transfer of the copyright to the publisher.
Informed Consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, Y., Huang, W., Ali, S.W. et al. Isolation, Identification, and Characterization of an Efficient Siderophore Producing Bacterium From Heavy Metal Contaminated Soil. Curr Microbiol 79, 227 (2022). https://doi.org/10.1007/s00284-022-02922-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00284-022-02922-5