Abstract
Dietary fiber has a potential to modulate the gut microbiota in sows. We hypothesized that a maternal diet rich in either high- or low-fermentable fiber during gestation and lactation influences Clostridioides difficile gut colonization in suckling piglets. Twenty sows were fed gestation and lactation diets enriched with either high-fermentable sugar beet pulp (SBP) or low-fermentable lignocellulose (LNC) fibers. C. difficile, toxin B (TcdB), fecal score, microbial abundance (16S-rDNA sequencing) and metabolites were measured in the feces from the sows and their piglets. C. difficile concentration was higher in piglets from the sows fed LNC than SBP along the study (P ≤ 0.05). Higher prevalence of C. difficile was noted in three-week-old piglets from sows fed LNC vs. SBP (45% vs. 0%, P = 0.001). TcdB prevalence was higher in six-day-old piglets from the sows fed LNC vs. SBP (60% vs. 17%, P = 0.009). In sows, fecal microbial metabolites were higher in SBP than LNC, while C. difficile concentration showed no difference. Higher microbial diversity Shannon index was noted in sows from SBP vs. LNC one week before parturition and at the parturition (P ≤ 0.05). Piglets from SBP vs. LNC tended to have higher microbial diversity Shannon index at two and three weeks of age. Diets enriched with high-fermentable fiber compared to low-fermentable fiber in sows reduced C. difficile colonization in their piglets. Susceptibility to colonization by C. difficile in neonatal piglets can be modulated by the sows’ diet, supporting the hypothesis of the early microbial programming in the offspring and the importance of the sow-piglet couple.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The association between sow and offspring is a critical factor for the microbial and immune development. After parturition, the neonatal piglet is exposed to an avalanche of diverse bacteria, which gradually populate the body surfaces including the gastrointestinal tract [1, 2]. Similar to humans, events taking place during gestation and after parturition may have an impact on the gut microecosystem in the offspring and intestinal health later in life [3,4,5]. Disruption of the natural microbial colonization process or perturbances of the intestinal ecosystem may enhance the susceptibility to gastrointestinal infections [6, 7]. The aspect of the sow–piglet association and its effect on early “microbial programming” and resilience to pathogens is gaining more attention [8]. On the contrary, the microbial association between mother and infant has already been widely studied [9, 10].
Gut bacteria require specific conditions for proliferation and metabolism in the host. The “windows of opportunity” for certain gut pathogens may depend on the age of the host or pathogens may benefit from gut microbial and immune dysbiosis [11, 12]. Clostridioides difficile is one of the pioneer colonizers in neonatal piglets and it has also been documented as a major cause of enteritis outbreaks in these animals [13]. In suckling piglets, C. difficile and its toxins can often be detected in feces up to two weeks after birth [14]. Both are occasionally found in weaned piglets and adult pigs indicating that sows could be a significant carrier of virulent C. difficile for their offspring [2]. Similar to piglets, toxigenic C. difficile is found in feces of human neonates, however, without any harm to the healthy host. Besides a hypothesis related to a lack of toxin receptors on infant gut epithelium, the toxin-neutralizing antibodies and other bioactive compounds present in colostrum and breastmilk, as well as the developing microbial colonization resistance, may be responsible for protection from C. difficile infection (CDI) in healthy infants [15]. Reducing the load of C. difficile inoculum in neonatal piglets and infants would be a promising approach to control C. difficile colonization and dissemination in the environment.
Diet is a strong modulating factor with a long-lasting impact on gut microbial ecosystem in pigs [1, 16]. Both, gestation and lactation periods seem to be the most promising stages for dietary interventions in sows to influence the fitness in the offspring. By altering the sows’ microbiota through nutritional factors, it may be possible to influence the piglets’ microbial development and health [17, 18]. Indeed, in humans, numerous data demonstrate associations between maternal nutritional status and microbiota on the infant gut microbiota, immune development and to a potential link to C. difficile [5, 9, 19, 20]. However, little is known about the impact of diet on the microbial association between sow and offspring and the establishment of the gut microbiota early in life. In this context, dietary fiber is an attractive feed ingredient that can influence the physiology and health of the sows and their offspring [21]. The source of fiber (i.e. soluble/high fermentable or insoluble/slowly fermentable) may shape the intestinal ecosystem of the sow and therefore of the offspring in different ways [22]. The influence of various types of fiber in the diet for lactating and gestating sows on C. difficile colonization in the offspring has not been studied yet. However, this approach could be a promising way to control the microbial colonization and metabolic patterns in sows and offspring and thereby protect against C. difficile expansion in suckling piglets. Therefore, we hypothesized that a sow diet rich in high- or low-fermentable fiber during gestation and lactation differently affects colonization of C. difficile in their piglets.
Material and Methods
Diets
Experimental gestation and lactation diets were formulated according to national recommendations to meet the sows’ requirements for nutrients [23]. The isoenergetic and isonitrogeneous diets provided high inclusion percentage of high-fermentable fiber source in form of sugar beet pulp (SBP; inclusion rate: 15% sugar beet pulp) or high percentage inclusion of low-fermentable fiber source in form of lignocellulose (LNC; inclusion rate: 15% lignocellulose), as shown in Table 1.
Feed Analyses
Weende proximate analysis (ash, crude fiber, crude protein, ether extract), neutral detergent fiber, acid detergent fiber, lignin and starch were determined using standard procedures VDLUFA (Verband Deutscher Landwirtschaftlicher Untersuchungs- und Forschungsanstalten) (VDLUFA III 3.1, VDLUFA III 4.1.1, VDLUFA III 6.1.4, VDLUFA III 8.1, VDLUFA III 5.1.1, VDLUFA III 7.2.1) [24].
Animals, Housing and Feeding
Twenty German Landrace sows (six multiparous, 14 gilts) were randomly allocated to the experimental feeding groups, with equal proportion of multiparous and gilt sows in each group. During gestation and lactation, the sows were kept without straw or other plant materials. Twenty gestating sows were kept in groups of 10 animals and housed spatially individually in farrowing pens one-week ante-partum until weaning. The farrowing occurred naturally without artificial induction. The sows were fed restrictively during gestation, while ad libitum during lactation. They were fed experimental gestation and lactation diets enriched with SBP (n = 10) or LNC (n = 10), as described above. Lactation diets were provided to sows three days after farrowing. Water was available to the animals ad libitum.
One week after farrowing, one sow from the SBP group and one sow from the LNC group were excluded from the trial due to post-farrowing complications, not related to the experimental diets.
Newborn piglets were balanced for sex and weight and four representative animals per sow (2 males, 2 females) were tagged and their fecal samples were collected during the suckling period. Suckling piglets were not provided with creep feed.
Sampling
Fresh fecal samples were collected from all the sows seven days before farrowing, at farrowing and seven days after farrowing. Feces from piglets were collected at day two, six, 10, 14, 21 and at weaning. Fecal samples were stored frozen at − 30 °C until analyzed. Fecal scores of the sows and piglets were recorded throughout the trial. The seven-scale “Bristol stool form scale” was adapted to assess the fecal score for all sow and all piglet samples [25], in which an additional score (“0”) was included if meconium was present in piglet feces. The fecal score was as follows: 0, meconium; 1, separate and hard; 2, hard but lumpy; 3, soft with cracks; 4, soft and smooth; 5, soft blobs; 6, soft and mushy; 7, watery (diarrheic). The experimental design is illustrated in Fig. 1.
Determination of C. difficile and Toxin B
Determination of C. difficile and toxin B in the feces was performed for all sow samples and for two of four piglets of each sow. C. difficile in the feces was quantified on C. difficile-ChromID selective agar plates (Biomerieux, France), as previously described [14].
To detect toxin B (TcdB) in the feces, enzyme-linked immunosorbent assay (ELISA) was carried out following the protocol of the ELISA commercial kit (tgcBIOMICS GmbH, Bingen, Germany) [14].
DNA Extraction
Fecal samples selected randomly from four sows/group/time point and from one of their piglets were used for DNA extraction and bacterial characterization by 16S-rDNA sequencing. DNA from fecal samples (0.25 g) obtained from piglets was extracted using the NucleoSpin DNA Stool kit (Macherey–Nagel, Düren, Germany) and from sows was extracted using the QIAamp Power Fecal Pro DNA kit (Qiagen, Hilden, Germany) following the manufacturer’s instructions. The extraction protocols were preceded by repeated bead-beating on a FastPrep-24™ 5G homogenizer (MP Biomedicals, LLC, Santa Ana, California, USA) to increase DNA extraction efficiency from spore-forming [26] and likely also Gram-positive bacteria.
Sequencing and Computational Data Analysis
DNA extracts were subjected to amplicon sequencing using an Illumina NextSeq500 sequencer (LGC, Berlin, Germany). Using a universal primer set, the 341F-785R V3-V4 region of the 16S rDNA was targeted and sequenced. The forward and reverse reads were combined using BBMerge tool (version 34.48) [27]. After demultiplexing, the resulting 16S-rDNA sequences of 108 samples were analyzed using QIIME2 pipeline [28] to determine microbial community profiles of each sample. Specifically, quality control was performed by DADA2 [29] routine within QIIME2. In the quality control process, chimeric sequences were removed, and regions of sequences with low-quality scores were truncated. The exact amplicon sequence variants (ASV) [30] and their respective counts in each sample were determined using DADA2. The ASV with total counts less than five sequences were excluded from further analysis to increase confidence of sequence reads. To account for the uneven distribution of sequences within samples, normalization was done through rarefication [31]. After rarefication to 10,000 reads, the sequence read depth of the samples was in saturation. Taxonomic assignment of the exact amplicon sequence variants was done using QIIME2’s feature classifier [32, 33] together with the SILVA SSU database [34] (release 132). Subsequent genus-level taxonomic profiles were generated based on the assignment of sequences and their corresponding counts. Bacterial taxa present in at least 10% of 108 samples were included in further statistical analysis on the relative abundances; they accounted for 172 taxa. Microbial diversity represented by Shannon index was calculated based on ASV using vegan package [35]. Shannon index was calculated from the following formula [36]: \(H^{\prime} = - \sum\limits_{i = 1}^{R} {pi\,\ln \,pi}\), where R is the observed number of species and pi is the proportional abundance of species i. The sequences have been deposited in a public repository: BioProject ID: PRJNA803022.
Analysis of Metabolites in Sow Feces
Feces were assessed for d- and l-lactate using high-performance liquid chromatography on an Agilent 1100 chromatograph, as previously described [37]. Ammonia in the feces was analyzed photometrically using the Berthelot reaction and extinction was measured using Tecan Sunrise™ microplate reader (Tecan Austria GmbH, Grödig, Austria). Fecal short-chain fatty acids (SCFA) were determined by gas chromatography on an Agilent 6890 gas chromatography system with flame ionization detector and autosampler (Agilent Technologies, Böblingen, Germany) [37]. Biogenic amines in the feces (putrescine, cadaverine, tyramine, histamine, spermidine and spermine) were analyzed with ion-exchange chromatography, as previously described [37].
Statistical Analyses
The data for C. difficile, TcdB and metabolites were analyzed by Mann–Whitney U test. Fisher's Exact test was used to test the percentages of positive values for C. difficile and TcdB. Differences for microbial diversity Shannon index and microbial abundance (16S-rDNA) were calculated by Mann–Whitney U test. Correlations between the concentrations of C. difficile and TcdB were assessed using the Spearman's correlation analysis procedure. Significant differences were considered at P ≤ 0.05. Statistical analyses were performed using the software SPSS 27.0 (SPSS Inc., Chicago, IL). The beeswarm plot was generated in RAWGraphs 2.0 beta [38].
Results
Bacterial Communities and Diversity Indices in Sow Feces
In the individual sow samples, we found between 45 and 100 bacterial taxa. The total number of identified bacterial taxa was 172, of which the 26 dominant taxa were displayed in stacked bar plots (Supplementary Figure S1). Here, taxa of Clostridium sensu stricto 1, Lactobacillus spp., Terrisporobacter spp., Romboutsia spp., and Streptococcus spp. predominated the gut microbiota of sows from both dietary groups throughout the sampling period. Sows fed LNC had a significantly higher abundance of Terrisporobacter spp. in their feces, as compared to sows fed SBP 30 days before farrowing (P = 0.029). In addition, in this same gestation period, there was a trend for a higher abundance of Bifidobacterium spp. in the feces of sows fed SBP vs. LNC (P = 0.057). One week before the farrowing, the abundance of sequences belonging to Muribaculaceae family was slightly increased in sows fed SBP vs. LNC (P = 0.057). At farrowing, the abundance of Maribaculaceae increased significantly (P = 0.029) whereas sequences belonging to Ruminococcaceae family (P = 0.057), Clostridium sensu stricto 1 (P = 0.057) showed a trend for an increase in abundance in sows fed SBP vs. LNC. On the contrary, the abundance of Lactobacillus spp. showed an increasing trend in sows fed LNC vs. SBP at the farrowing. One week after farrowing, the abundance of Lactobacillus spp. showed a continuous trend for an increase in sows fed LNC vs. SBP (P = 0.057).
At farrowing, Shannon index was significantly higher in sows from SBP vs. LNC group (3.8 ± 0.10 vs. 2.7 ± 0.34, P = 0.029, respectively).
Fecal Consistency and C. difficile Shedding in Sows
Feces of the sows from both feeding groups had a physiological consistency (score 3) throughout the gestation and lactation periods and constipation or diarrhea was not observed. Feces of sows fed SBP diet were moister and darker in color than feces of sows fed LNC diet.
C. difficile was not detectable one week before farrowing in any of the sows. It was determined at the farrowing day in only one sow from SBP group (log10 2.30 CFU/g) and in one sow from LNC group (log10 2.00 CFU/g). C. difficile was detected seven days post-partum in all the sows from SBP group (median log10 3.5 CFU/g, min–max 2.0–4.5) and in all the sows from LNC group (median log10 3.2 CFU/g, min–max 2.6–4.5) (P = 0.258). TcdB was not detected in any of the sows during the periparturient period.
Fecal Metabolite Patterns in Sow Feces
Results on fecal metabolite patterns in sows during periparturient period are shown in Table 2. One week before farrowing, levels of spermidine were significantly higher in feces of sows fed SBP, as compared to sows fed LNC (P < 0.001). On the farrowing day, significantly higher concentrations of fecal propionate (P = 0.033), i-butyrate (P < 0.001), ammonia (P = 0.043), cadaverine (P = 0.028) and spermine (P = 0.001) were detected in feces of sows fed SBP, as compared to sows fed LNC. One week after farrowing, a trend for higher levels of acetate (P = 0.053), propionate (P = 0.053), as well as significantly higher i-butyrate (P = 0.013), i-valerate (P = 0.043), ammonia (P = 0.008) and spermidine (P = 0.008) were found in feces of sows fed SBP, as compared to sows fed LNC.
Piglet Fecal Score
The fecal score of the study piglets is shown in Fig. 2. Two days after birth, majority of piglets from SBP and LNC groups defecated meconium or soft to watery feces. At six and 10 days of life, majority of feces had a hard consistency in both groups. Fourteen- and 21-day-old piglets from the LNC group presented more constipated stools than piglets from the SBP group. At weaning, piglets from SBP group had softer feces than from the LNC group.
Fecal score in piglet feces whose mother sows were fed diets containing high-fermentable sugar beet pulp (light-grey points) or low-fermentable lignocellulose (dark-grey points) fibers during gestation and lactation. Each dot represents an individual piglet faecal sample. The seven-scale “Bristol stool form scale” was adapted to assess the fecal score for all sow and all piglet samples, in which an additional score (“0”) was included if meconium was present in piglet feces. The fecal score was as follows: 0, meconium; 1, separate and hard; 2, hard but lumpy; 3, soft with cracks; 4, soft and smooth; 5, soft blobs; 6, soft and mushy; 7, watery (diarrheic)
Colonization of Piglets by C. difficile
C. difficile was already detected in two-day-old piglets from LNC group (log10 2.7 CFU/g) but not in piglets from SBP group. Concentrations of C. difficile were significantly lower in six-, 10- and 14-day-old piglets from the sows fed SBP than LNC diets (log10 6.3 CFU/g vs. log10 7.0 CFU/g, P = 0.034; log10 3.8 CFU/g vs. log10 5.6 CFU/g, P = 0.024; log10 3.4 CFU/g vs. log10 4.4 CFU/g, P = 0.010, respectively). In three-week-old piglets from the LNC group C. difficile concentration was log10 3.7 CFU/g, while the bacterium was not detected in piglets from SBP group. At weaning, concentrations of C. difficile were lower in piglets from SBP as compared to LNC group (log10 1.9 CFU/g vs. log10 2.2 CFU/g, P = 0.098) (Fig. 3).
Concentration of C. difficile (log10 CFU/g) in piglet feces whose mother sows were fed diets containing high-fermentable sugar beet pulp (SBP) or low-fermentable lignocellulose (LNC) fibers during gestation and lactation. Circles indicate outliers. Sample number (SBP/LNC) in each boxplot: 2d: 0/2; 6d: 13/13; 10d: 15/16; 14d: 8/13; 21d: 0/9; weaning: 8/12
The percentage of piglets shedding C. difficile tended to be higher in piglets from the sows fed LNC than SBP diets along the trial (Table 3). At day 21, significantly lower percentage of piglets from the sows fed SBP than LNC shed C. difficile in their feces (0% vs. 45.0%, P = 0.001).
Toxin B in Piglets
TcdB was detected already in feces from two-day-old piglets, however at a low concentration in both feeding groups (log10 1.3 ng/g vs. log10 0.5 ng/g, P = 0.200). The concentration of TcdB in six-day-old piglets was log10 1.0 ng/g and log10 1.0 ng/g in piglets from SBP and LNC group (P = 0.840), respectively. At 10 days of age TcdB was lower in piglets from SBP, as compared to LNC group (log10 1.3 ng/g vs. log10 1.6 ng/g, P = 0.629). Fourteen-day-old piglets from SBP and LNC groups carried log10 1.3 ng/g and log10 0.9 ng/g of TcdB (P = 0.800), respectively. At 3 weeks of age the TcdB was not detected in piglets from SBP group, but it was determined at a concentration of log10 0.3 ng/g in piglets from LNC group (Fig. 4).
Concentration of TcdB (log10 ng/g) in piglet feces whose mother sows were fed diets containing high-fermentable sugar beet pulp (SBP) or low-fermentable lignocellulose (LNC) fibers during gestation and lactation. Sample number (SBP/LNC) in each boxplot: 2d: 3/2; 6d: 3/12; 10d: 3/4; 14d: 4/2; 21d: 0/2; weaning: 0/0
At six days of age, significantly more piglets from the LNC group were positive for TcdB, as compared to piglets from the SBP group (60.0% vs. 16.7%, P = 0.009) (Table 4).
Concentration of C. difficile was positively correlated with the concentration of TcdB (r = 0.568, n = 23, P = 0.005).
Bacterial Communities and Diversity Indices in Piglet Feces
In the individual piglet fecal samples, we found between 12 and 113 bacterial taxa. The total number of identified bacterial taxa was 172, of which the 26 dominant taxa were displayed in stacked bar plots. (Supplementary Figure S2). Here, taxa of Lactobacillus spp., Clostridium sensu stricto 1 and sequences belonging to Muribaculaceae family predominated the gut microbiota of piglets. A significantly higher abundance of Lachnoclostridium spp. (P = 0.029) and Bifidobacterium spp. (P = 0.029) were found in the feces of 14-day-old piglets from sows fed LNC vs. SBP. Here, there was trend for a higher abundance of Prevotella spp. (P = 0.057) in the piglets from sows fed SBP vs. LNC. One week later, taxa of Coprococcus 3 spp. (P = 0.029) and Terrisporobacter spp. (P = 0.029) predominated in the feces of piglets from sows fed SBP vs. LNC while the abundance of Escherichia-Schigella taxon (P = 0.029) was significantly higher in piglets from sows fed LNC vs. SBP. At weaning, a significantly higher abundance of Terrisporobacter spp. (P = 0.029) and a trend for a higher abundance of Coprococcus 3 spp. (P = 0.057) taxa were found in piglets from sows fed SBP vs. LNC. On the contrary, a significantly higher abundance of Ruminococcus spp. (P = 0.029) was detected in piglets from sows fed LNC vs. SBP.
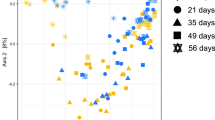
In piglets, microbial diversity represented by Shannon index gradually increased as the animals aged (Fig. 5). At 2 weeks of age, there was a trend for a higher bacterial Shannon diversity index in piglets from sows fed SBP vs. LNC (P = 0.057).
Diversity shown as Shannon index using the relative abundance of ASV in piglet feces (n = 4/age/group) whose mother sows (n = 4/age/group) were fed diets containing high-fermentable sugar beet pulp (SBP) or low-fermentable lignocellulose (LNC) fibers during gestation and lactation, as analyzed by the 16S-rDNA sequencing. Light-grey bars represent SBP, while dark-grey bars represent LNC
Discussion
The influence of the mother on the offspring ontogeny and health by different dietary means in animals and humans has gained increasing interest. It has been demonstrated that the association between mother and infant is important for the maturation of the gut microbiota and immune system since events taking place during perinatal period have important implications in early life and adulthood [19, 39]. However, little is known about the mother–offspring association in pigs, especially considering microbial imprinting during the neonatal period. Moreover, sow-originated factors may contribute to a protection against microbial pathogens including C. difficile in piglets. Indeed, sow nutrition during pregnancy and lactation has been shown to influence the health and wellbeing of their piglets [17, 40].
Here, we assessed the effect of sows’ diet enriched in highly- or low-fermentable fiber sources on the colonization of piglets by C. difficile. In this study, the sows were fed diets containing higher concentrations of either high-fermentable sugar beet pulp or low-fermentable lignocellulose during gestation and lactation periods. Our results show that those suckling piglets whose mothers consumed diets enriched with sugar beet pulp had lower concentrations of C. difficile in their feces compared to piglets whose mothers consumed diets enriched with lignocellulose. Suckling piglets of the sows fed sugar beet pulp were also less likely to be colonized by C. difficile along the suckling period, as compared to their counterparts. Lower concentrations of toxin B were detected in piglets from sows fed sugar beet pulp, as compared to lignocellulose. In addition, the prevalence of piglets in which toxin B was detected was lower in one-week-old animals from sows fed sugar beet pulp compared to lignocellulose.
C. difficile colonizes the piglets gut at birth and its concentration increases rapidly during the first week of life [14]. Among C. difficile, virulent types are present which are able to produce toxins such as toxin A and/or B, leading to gut intoxication and CDI progress [41]. In our study, the microbial diversity as assessed by Shannon index, increased with the piglets’ age. The developing and yet immature gut microbiota characterized by a low diversity, as we observed in the present and earlier studies, may open a niche for C. difficile successful colonization in suckling piglets [2]. However, due to different fecal sample number for C. difficile and microbiota determination, the analysis of a direct effect of microbiota on C. difficile could not be performed. Further, any disruption of the natural colonization process or perturbances of the intestinal ecosystem could increase the chances of piglets developing CDI [41]. In a dynamic and complex gut ecosystem, certain bacterial groups may be in intimate contact with each other, entering different ecological dependences. Likewise, infants treated with antimicrobials or suffering from bacterial or viral gut infections are at higher risk of developing CDI [42]. In suckling piglets, CDI is characterized by diarrhea or constipation, increased body temperature and a presence of C. difficile and its toxins in feces [13]. In this study, we quantified toxin B only, since more CDI outbreaks are associated with C. difficile producing either both toxins or toxin B, rather than toxin A only [43].
A limited number of studies assessed the impact of dietary intervention on C. difficile colonization in animals. For instance, atherogenic, axenic or elemental diets fed to hamsters and mice caused an increased C. difficile proliferation and toxin synthesis in the gut, which resulted in CDI development and lower survival rates of the animals [44,45,46,47]. In humans, previous reports demonstrated increased levels and prevalence of C. difficile in formula fed vs. breastfed infants [5]. Moreover, C. difficile was more often detected in infants from high-income vs. low-income countries [48], suggesting that a maternal diet, often high in processed foods in high-income countries and high in fiber in low-income countries, can be directly or indirectly related to C. difficile colonization in infants. In our study, the results clearly suggest a protective impact of the addition of high fermentable fibers over low fermentable fibers in sow’s feeds during gestation and lactation periods against the colonization of piglets by C. difficile. Specifically, a more diverse microbiota was found during periparturient period in sows fed sugar beet pulp, as compared to sows fed lignocellulose. Following this finding, suckling piglets from the sows fed sugar beet pulp compared to lignocellulose tended to have a higher microbial diversity, supporting the hypothesis of the mother–offspring microbial programming and microbial diversity being associated with C. difficile colonization [2, 8]. Similarly, a previous study showed that highly fermentable non-starch-polysaccharides, such as inulin added to the diet can modulate certain bacterial groups (as analyzed by qPCR) of gestating and lactating sows and their suckling piglets [49]. Inclusion of certain carbohydrates which are not digestible for pigs but for gut microorganisms is known to influence the microbial metabolic activity [37]. Here, metabolites were assessed in sow feces collected during the periparturient period i.e. one week ante-partum, at the farrowing and one week post-partum, as in this period the sow feces have a direct contact with a newborn piglet which may potentially influence the early microbial programming in the offspring. We found that the addition of sugar beet pulp to the sows’ diet during gestation and lactation increased production of SCFA, certain biogenic amines and ammonia in the feces during the periparturient period, as compared to sows fed diets enriched with lignocellulose. Sugar beet pulp has been shown to be more easily degradable by the gut microbiota, due to its higher solubility and accessibility to microbial enzymes than lignocellulose, resulting in a production of higher levels of microbial metabolites [50]. Previous in vitro studies have demonstrated a negative impact of high levels of SCFA and low pH on C. difficile growth and toxin production [51, 52]. Clinical data demonstrate that butyrate-producing anaerobic bacteria were significantly depleted in the CDI patients [6]. Recently, stool samples from patients with CDI had lower valerate concentrations, which were restored after fecal microbial transplantation and additionally, in chemostat models valerate decreased vegetative growth of C. difficile [53]. Although an increased microbial metabolic activity in the sows fed sugar beet pulp did not influence C. difficile shedding by the sows compared to sows fed lignocellulose, a higher concentration of metabolites in the sows’ feces may have influenced C. difficile colonization in their piglets. Pigs are known for coprophagy and nursing piglets have a constant contact with sow’s feces after birth which may facilitate the influence of sows fecal microecology on their offspring [54]. Considering the yet immature gut microecosystem in neonatal piglets and its vulnerability to environmental factors, changes in microbial composition accompanied by higher concentrations of SCFA in the feces of sows fed sugar beet pulp may have reduced C. difficile proliferation in suckling piglets. To understand these specific relationships, targeted in vitro and ex vivo approaches may be necessary.
Farrowing is a stressful period for mammals, which has a negative impact on physiology, immune system and microbial diversity [55, 56]. Indeed, we have previously observed shifts in the gut microbiota composition in sows during periparturient period and such changes may possibly lead to an increased susceptibility to certain infections [2]. Similarly, microbial dysbiosis accompanied by an increase in proinflammatory cytokines and energy loss has been observed in healthy pregnant women [55, 57]. Thus, specific dietary strategies could offer an opportunity to influence the gut ecology and health of the host. It is noteworthy that one week after farrowing, all sows had detectable levels of C. difficile in their feces. Interestingly, since C. difficile rapidly colonizes one-week-old neonatal piglets, it is very likely that a high load of C. difficile excreted with the piglet’s feces re-inoculated the sows due to constant sow-piglet contact and coprophagy during the nursing period [54]. The proposed “re-inoculation” phenomenon of C. difficile to the sows, could possibly possess a risk of CDI, if in addition the sows were treated with antimicrobials which are a contributing factor to CDI development in young pigs or in humans, as reviewed previously [8]. If the “re-inoculation” phenomenon were true, it would mean that not only a sow could influence the microbial ecosystem of their piglets but also vice versa. Such observation may have important consequences for the health of the sows during periparturient period and should be investigated in future studies. Likewise, high prevalence of toxigenic C. difficile observed in infant feces could contribute to pathogen dissemination in a community and possess a risk of infection in adults suffering from gut microbial dysbiosis [58]. This can have important public health implications and should be studied in more detail.
The awareness of the prenatal and postnatal environment is fundamental for the early microbial and immune programming. Mammal offspring are in the intimate contact with their mothers already in utero following the nursing period [16]. In humans, it is known that the maternal microbiota, immune system and metabolism have a profound impact on infant development [59]. In intensive production systems, neonatal piglets are more vulnerable to stress and gut dysbiosis [60]. Identifying the beneficial effects of dietary components and their roles during gestation and lactation may have promising implications in microbiota programming of the offspring, controlling of pathogen colonization and dissemination, and reducing the risk of diseases. Finally, the opportunity to improve piglet health through the sow-offspring association by dietary means offers an attractive approach to control piglet’s resilience to gut pathogens, such as C. difficile.
Taken together, sow’s diets enriched with highly fermentable sugar beet pulp, compared to low fermentable lignocellulose dietary fibers during gestation and lactation reduced C. difficile shedding in suckling piglets. Susceptibility to colonization by gut pathogens, such as C. difficile in neonatal piglets can be influenced by the sows’ nutritional factors supporting the phenomenon of the mother–offspring early microbial programming.
Abbreviations
- C. difficile :
-
Clostridioides difficile
- CDI:
-
Clostridioides difficile Infection
- TcdB:
-
Toxin B
- SBP:
-
Sugar beet pulp
- LNC:
-
Lignocellulose
- ELISA:
-
Enzyme-linked immunosorbent assay
- ASV:
-
Amplicon sequence variants
References
Bian G, Ma S, Zhu Z et al (2016) Age, introduction of solid feed and weaning are more important determinants of gut bacterial succession in piglets than breed and nursing mother as revealed by a reciprocal cross-fostering model. Environ Microbiol 18:1566–1577. https://doi.org/10.1111/1462-2920.13272
Grześkowiak D, Zentek V (2019) Developing gut microbiota exerts colonisation resistance to clostridium (syn. Clostridioides) difficile in piglets. Microorganisms 7:218. https://doi.org/10.3390/microorganisms7080218
Li Y, Liu H, Zhang L et al (2020) Maternal dietary fiber composition during gestation induces changes in offspring antioxidative capacity, inflammatory response, and gut microbiota in a sow model. Int J Mol Sci. https://doi.org/10.3390/ijms21010031
Theil PK, Lauridsen C, Quesnel H (2014) Neonatal piglet survival: impact of sow nutrition around parturition on fetal glycogen deposition and production and composition of colostrum and transient milk. Animal 8:1021–1030. https://doi.org/10.1017/S1751731114000950
Grześkowiak Ł, Grönlund M-M, Beckmann C et al (2012) The impact of perinatal probiotic intervention on gut microbiota: double-blind placebo-controlled trials in Finland and Germany. Anaerobe 18:7–13. https://doi.org/10.1016/j.anaerobe.2011.09.006
Antharam VC, Li EC, Ishmael A et al (2013) Intestinal dysbiosis and depletion of butyrogenic bacteria in Clostridium difficile infection and nosocomial diarrhea. J Clin Microbiol 51:2884–2892. https://doi.org/10.1128/JCM.00845-13
Sun J, Du L, Li XL et al (2019) Identification of the core bacteria in rectums of diarrheic and non-diarrheic piglets. Sci Rep 9:1–10. https://doi.org/10.1038/s41598-019-55328-y
Grześkowiak ŁM, Pieper R, Huynh HA et al (2019) Impact of early-life events on the susceptibility to Clostridium difficile colonisation and infection in the offspring of the pig. Gut Microbes 10:251–259. https://doi.org/10.1080/19490976.2018.1518554
Grönlund M, Grzeskowiak Ł, Isolauri E, Salminen S (2011) Influence of mother’s intestinal microbiota on gut colonization in the infant. Gut Microbes 2:1–7
Gueimonde M, Sakata S, Kalliomäki M et al (2006) Effect of maternal consumption of lactobacillus GG on transfer and establishment of fecal bifidobacterial microbiota in neonates. J Pediatr Gastroenterol Nutr 42:166–170. https://doi.org/10.1097/01.mpg.0000189346.25172.fd
Friedemann M (2009) Epidemiology of invasive neonatal Cronobacter (Enterobacter sakazakii) infections. Eur J Clin Microbiol Infect Dis 28:1297–1304. https://doi.org/10.1007/s10096-009-0779-4
Grześkowiak Ł, Martínez-Vallespín B, Dadi TH et al (2018) Formula feeding predisposes neonatal piglets to Clostridium difficile gut infection. J Infect Dis 217:1442–1452. https://doi.org/10.1093/infdis/jix567
Songer JG, Anderson MA (2006) Clostridium difficile: an important pathogen of food animals. Anaerobe 12:1–4. https://doi.org/10.1016/j.anaerobe.2005.09.001
Grześkowiak Ł, Zentek J, Vahjen W (2016) Determination of the extent of Clostridium difficile colonisation and toxin accumulation in sows and neonatal piglets. Anaerobe 40:5–9. https://doi.org/10.1016/j.anaerobe.2016.04.012
Rolfe RD, Song W (1995) Immunoglobulin and non-immunoglobulin components of human milk inhibit Clostridium difficile toxin A-receptor binding. J Med Microbiol 42:10–19. https://doi.org/10.1099/00222615-42-1-10
Everaert N, Van Cruchten S, Weström B et al (2017) A review on early gut maturation and colonization in pigs, including biological and dietary factors affecting gut homeostasis. Anim Feed Sci Technol 233:89–103. https://doi.org/10.1016/j.anifeedsci.2017.06.011
Shang Q, Liu H, Liu S et al (2019) Effects of dietary fiber sources during late gestation and lactation on sow performance, milk quality, and intestinal health in piglets. J Anim Sci 97:4922–4933. https://doi.org/10.1093/jas/skz278
Loisel F, Farmer C, Ramaekers P, Quesnel H (2013) Effects of high fiber intake during late pregnancy on sow physiology, colostrum production, and piglet performance. J Anim Sci 91:5269–5279. https://doi.org/10.2527/jas.2013-6526
Kalliomäki M, Salminen S, Arvilommi H et al (2001) Probiotics in primary prevention of atopic disease: a randomised placebo-controlled trial. Lancet 357:1076–1079
Collado MC, Isolauri E, Laitinen K, Salminen S (2010) Effect of mother ’s weight on infant ’s microbiota acquisition, composition, and activity during early infancy : a prospective follow-up study initiated in early pregnancy. Am J Clin Nutr 92:1023–1030. https://doi.org/10.3945/ajcn.2010.29877.Overweight
Krogh U, Bruun TS, Amdi C et al (2015) Colostrum production in sows fed different sources of fiber and fat during late gestation. Can J Anim Sci 95:211–223. https://doi.org/10.4141/CJAS-2014-060
Li Y, Zhang L, Liu H et al (2019) Effects of the ratio of insoluble fiber to soluble fiber in gestation diets on sow performance and offspring intestinal development. Animals 9:1–17. https://doi.org/10.3390/ani9070422
GfE, (2006) Empfehlungen zur Energie- und Nährstoffversorgung von Schweinen. DLG Verlag, Frankfurt
Naumann C, Bassler R, Seibold R, Barth C (1976) Methodenbuch. Band III, Band III. VDLUFA - Verlag, Darmstadt
Lewis SJ, Heaton KW (1997) Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol 32:920–924. https://doi.org/10.3109/00365529709011203
Grześkowiak Ł, Zentek J, Vahjen W (2016) Physical pre-treatment improves efficient DNA extraction and qPCR sensitivity from Clostridium difficile spores in faecal swine specimens. Curr Microbiol 73:727–731. https://doi.org/10.1007/s00284-016-1123-8
Bushnell B, Rood J, Singer E (2017) BBMerge: accurate paired shotgun read merging via overlap. PLoS ONE 12:1–15. https://doi.org/10.1371/journal.pone.0185056
Bolyen E, Rideout JR, Dillon MR et al (2018) QIIME 2: reproducible, interactive, scalable, and extensible microbiome data science. PeerJ Prepr 6:e27295v1. https://doi.org/10.7287/peerj.preprints.27295v1
Callahan BJ, McMurdie PJ, Rosen MJ et al (2016) DADA2: high-resolution sample inference from Illumina amplicon data. Nat Methods 13:581. https://doi.org/10.1038/nmeth.3869
Callahan BJ, McMurdie PJ, Holmes SP (2017) Exact sequence variants should replace operational taxonomic units in marker-gene data analysis. ISME J 11:2639
Weiss S, Xu ZZ, Peddada S et al (2017) Normalization and microbial differential abundance strategies depend upon data characteristics. Microbiome 5:27. https://doi.org/10.1186/s40168-017-0237-y
Bokulich NA, Kaehler BD, Rideout JR et al (2018) Optimizing taxonomic classification of marker-gene amplicon sequences with QIIME 2’s q2-feature-classifier plugin. Microbiome 6:90. https://doi.org/10.1186/s40168-018-0470-z
Pedregosa F, Varoquaux G, Gramfort A et al (2011) Scikit-learn: machine learning in Python. J Mach Learn Res 12:2825–2830
Yilmaz P, Parfrey LW, Yarza P et al (2014) The SILVA and “all-species Living Tree Project (LTP)” taxonomic frameworks. Nucleic Acids Res 42:D643–D648. https://doi.org/10.1093/nar/gkt1209
Oksanen AJ, Blanchet FG, Friendly M et al (2019) Vegan. Encycl Food Agric Ethics. https://doi.org/10.1007/978-94-024-1179-9_301576
Spellerberg IF, Fedor PJ (2003) A tribute to Claude-Shannon (1916–2001) and a plea for more rigorous use of species richness, species diversity and the “Shannon-Wiener” Index. Glob Ecol Biogeogr 12:177–179. https://doi.org/10.1046/j.1466-822X.2003.00015.x
Pieper R, Boudry C, Bindelle J et al (2014) Interaction between dietary protein content and the source of carbohydrates along the gastrointestinal tract of weaned piglets. Arch Anim Nutr 68:263–280. https://doi.org/10.1080/1745039X.2014.932962
Mauri M, Elli T, Caviglia G, et al (2017) RAWGraphs: a visualisation platform to create open outputs. In: Proceedings of the 12th biannual conference on Italian SIGCHI Chapter. Association for Computing Machinery, New York, NY, USA
Marko K, Seppo S, Tuija P, Arvilommi Heikki IE (2003) Probiotics and prevention of atopic disease : 4-year follow-up of a randomised placebo-controlled trial For personal use. Only reproduce with permission from The Lancet Publishing Group. Lancet 361:1869–1871
Quesnel H, Meunier-Salaün MC, Hamard A et al (2009) Dietary fiber for pregnant sows: influence on sow physiology and performance during lactation. J Anim Sci 87:532–543. https://doi.org/10.2527/jas.2008-1231
Grzeskowiak L, Martinez-Vallespin B, Dadi TH et al (2017) Formula-feeding predisposes neonatal piglets to Clostridium difficile gut infection. J Infect Dis. https://doi.org/10.1093/infdis/jix567
Lees EA, Miyajima F, Pirmohamed M, Carrol ED (2016) The role of Clostridium difficile in the paediatric and neonatal gut: a narrative review. Eur J Clin Microbiol Infect Dis 35:1047–1057. https://doi.org/10.1007/s10096-016-2639-3
Drudy D, Fanning S, Kyne L (2007) Toxin A-negative, toxin B-positive Clostridium difficile. Int J Infect Dis 11:5–10. https://doi.org/10.1016/j.ijid.2006.04.003
Mahe S, Corthier G, Dubos F (1987) Effect of various diets on toxin production by two strains of Clostridium difficile in gnotobiotic mice. Infect Immun 55:1801–1805
Frankel WL, Choi DM, Zhang W et al (1994) Soy fiber delays disease onset and prolongs survival in experimental Clostridium difficile ileocecitis. JPEN J Parenter Enteral Nutr 18:55–61
Blankenship-Paris TL, Walton BJ, Hayes YO, Chang J (1995) Clostridium difficile infection in hamsters fed an atherogenic diet. Vet Pathol 32:269–273
Iizuka M, Itou H, Konno S et al (2004) Elemental diet modulates the growth of Clostridium difficile in the gut flora. Aliment Pharmacol Ther 20(Suppl 1):151–157. https://doi.org/10.1111/j.1365-2036.2004.01969.x
Grześkowiak Ł, Collado MC, Mangani C et al (2012) Distinct gut microbiota in Southeastern African and Northern European infants. J Pediatr Gastroenterol Nutr 54:812–816. https://doi.org/10.1097/MPG.0b013e318249039c
Paßlack N, Vahjen W, Zentek J (2015) Dietary inulin affects the intestinal microbiota in sows and their suckling piglets. BMC Vet Res 11:51. https://doi.org/10.1186/s12917-015-0351-7
Pieper R, Kroger S, Richter JF et al (2012) Fermentable fiber ameliorates fermentable protein-induced changes in microbial ecology, but not the mucosal response, in the colon of piglets. J Nutr 142:661–667. https://doi.org/10.3945/jn.111.156190
Karlsson S, Lindberg A, Norin E et al (2000) Toxins, butyric acid, and other short-chain fatty acids are coordinately expressed and down-regulated by cysteine in Clostridium difficile. Infect Immun 68:5881–5888. https://doi.org/10.1128/IAI.68.10.5881-5888.2000
May T, Mackie RI, Fahey GC et al (1994) Effect of fiber source on short-chain fatty acid production and on the growth and toxin production by Clostridium difficile. Scand J Gastroenterol 29:916–922. https://doi.org/10.3109/00365529409094863
McDonald JAK, Mullish BH, Pechlivanis A et al (2018) Inhibiting growth of Clostridioides difficile by restoring valerate, produced by the intestinal microbiota. Gastroenterology 155:1495-1507.e15. https://doi.org/10.1053/j.gastro.2018.07.014
Whatson TS, Bertram JM (1983) Some observations on mother-infant interactions in the pig (Sus scrofa). Appl Anim Ethol 9:253–261. https://doi.org/10.1016/0304-3762(83)90005-6
Koren O, Goodrich JK, Cullender TC et al (2012) Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell 150:470–480. https://doi.org/10.1016/j.cell.2012.07.008
Nuriel-Ohayon M, Neuman H, Koren O (2016) Microbial changes during pregnancy, birth, and infancy. Front Microbiol 7:1–13. https://doi.org/10.3389/fmicb.2016.01031
Collado MC, Isolauri E, Laitinen K, Salminen S (2008) Distinct composition of gut microbiota during pregnancy in overweight and normal-weight women. Am J Clin Nutr 88:894–899
Smits WK, Lyras D, Lacy DB et al (2016) Clostridium difficile infection. Nat Rev Dis Prim 2:16020. https://doi.org/10.1038/nrdp.2016.20
Milani C, Duranti S, Bottacini F et al (2017) The first microbial colonizers of the human gut: composition, activities, and health implications of the infant gut microbiota. Microbiol Mol Biol Rev 81:1–67. https://doi.org/10.1128/mmbr.00036-17
Pluske JR, Turpin DL, Kim JC (2018) Gastrointestinal tract (gut) health in the young pig. Anim Nutr 4:187–196. https://doi.org/10.1016/j.aninu.2017.12.004
Knudsen KEB (1997) Carbohydrate and lignin contents of plant materials used in animal feeding. Anim Feed Sci Technol 67:319–338. https://doi.org/10.1016/S0377-8401(97)00009-6
Acknowledgements
We thank the animal caretakers for help in animal handling and sampling and P. Huck, A. Kriesten, K. Schröter, L. Ebersbach, C. Kipar, M. Schwebig, M. Moessle and H. Nisic for help in feed and fecal sample analyzes. Dr. R. Pieper is acknowledged for advice on the concept of the experimental feeds.
Funding
Open Access funding enabled and organized by Projekt DEAL. This study was financially supported by the German Research Foundation (Deutsche Forschungsgemeinschaft. DFG) Grant GR 5107/2-1.
Author information
Authors and Affiliations
Contributions
Conceptualization: ŁG, WV and JZ; animal experiment and data collection: ŁG, E-MS, BM-V, AGW and KM; formal analysis and original draft preparation: ŁG; review and editing: E-MS, BM-V, AGW, KM, WV and JZ. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
No potential conflict of interest was reported by the authors. All authors have read and agreed to the published version of the manuscript.
Ethical Approval
The institutional and national guidelines for the care and use of animals were followed and the study was approved by the State Office of Health and Social Affairs ‘Landesamt für Gesundheit und Soziales Berlin’ (LAGeSo Reg. G0112/19). This study was conducted in the experimental pig facilities of the Institute of Animal Nutrition at the Freie Universität Berlin in Berlin in Germany. The institute has its own sow breeding facility and piglets are regularly breed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Grześkowiak, Ł., Saliu, EM., Martínez-Vallespín, B. et al. Fiber Composition in Sows’ Diets Modifies Clostridioides difficile Colonization in Their Offspring. Curr Microbiol 79, 154 (2022). https://doi.org/10.1007/s00284-022-02848-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00284-022-02848-y