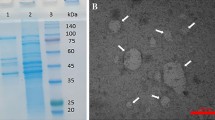
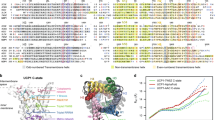
Abstract
Uncoupling protein-1 (UCP1), located at the inner membrane of mitochondria, is expressed primarily in brown adipose tissue and mediates the permeability of protons through the inner mitochondrial membrane. This research examines whether human UCP1 can uncouple oxidative phosphorylation in E. coli. Recombinant human UCP1 that includes an N terminus signal peptide for the bacterial inner membrane was expressed in E. coli. Our testing showed that UCP1 functions as a proton transporter in the bacterial membrane, increasing its permeability, decrease ATP synthesis at neutral pH and reducing the viability of E. coli in markedly acidic environments. These results suggest that UCP1 can uncouple oxidative phosphorylation in E. coli. The decreased acid resistance (AR) of E. coli with UCP1 expressed in the membranes confirmed that oxidative phosphorylation plays a role in AR through the pumping of protons to regulate the intracellular pH, and demonstrate that UCP1 can be used as an uncoupler protein for bacterial metabolic research.





Similar content being viewed by others
References
A Fedorenko PV Lishko Y Kirichok 2012 Mechanism of fatty-acid-dependent UCP1 uncoupling in brown fat mitochondria Cell 151 2 400 413 https://doi.org/10.1016/j.cell.2012.09.010
ET Chouchani L Kazak MP Jedrychowski G Lu BK Erickson J Szpyt KA Pierce D Laznik-Bogoslavski R Vetrivelan CB Clish 2016 Mitochondrial ROS regulate thermogenic energy expenditure and sulfenylation of UCP1 Nature 532 7597 112 116 https://doi.org/10.1038/nature17399
AM Bertholet Y Kirichok 2017 UCP1: a transporter for H(+) and fatty acid anions Biochimie 134 28 34 https://doi.org/10.1016/j.biochi.2016.10.013
KJ Chung A Chatzigeorgiou M Economopoulou R Garcia-Martin VI Alexaki I Mitroulis M Nati J Gebler T Ziemssen SE Goelz 2017 A self-sustained loop of inflammation-driven inhibition of beige adipogenesis in obesity Nat immunol 18 6 654 664 https://doi.org/10.1038/ni.3728
MK Nohr N Bobba B Richelsen S Lund SB Pedersen 2017 Inflammation downregulates UCP1 expression in brown adipocytes potentially via SIRT1 and DBC1 interaction Int J Mol Sci https://doi.org/10.3390/ijms18051006
JP Abrahams AG Leslie R Lutter JE Walker 1994 Structure at 2.8 A resolution of F1-ATPase from bovine heart mitochondria Nature 370 6491 621 628 https://doi.org/10.1038/370621a0
T Hoang MD Smith M Jelokhani-Niaraki 2013 Expression, folding, and proton transport activity of human uncoupling protein-1 (UCP1) in lipid membranes: evidence for associated functional forms J Biol Chem 288 51 36244 36258 https://doi.org/10.1074/jbc.M113.509935
L Kazak ET Chouchani IG Stavrovskaya GZ Lu MP Jedrychowski DF Egan M Kumari X Kong BK Erickson J Szpyt 2017 UCP1 deficiency causes brown fat respiratory chain depletion and sensitizes mitochondria to calcium overload-induced dysfunction Proc Natl Acad Sci USA 114 30 7981 7986 https://doi.org/10.1073/pnas.1705406114
C Shibata T Ehara K Tomura K Igarashi H Kobayashi 1992 Gene structure of Enterococcus hirae (Streptococcus faecalis) F1F0-ATPase, which functions as a regulator of cytoplasmic pH J Bacteriol 174 19 6117 6124 https://doi.org/10.1128/jb.174.19.6117-6124.1992
Y Sun T Fukamachi H Saito H Kobayashi 2012 Respiration and the F(1)Fo-ATPase enhance survival under acidic conditions in Escherichia coli PLoS ONE 7 12 e52577 https://doi.org/10.1371/journal.pone.0052577
N Kinoshita T Unemoto H Kobayashi 1984 Proton motive force is not obligatory for growth of Escherichia coli J Bacteriol 160 3 1074 1077 https://doi.org/10.1128/jb.160.3.1074-1077.1984
Y Sun T Fukamachi H Saito H Kobayashi 2011 ATP requirement for acidic resistance in Escherichia coli J Bacteriol 193 12 3072 3077 https://doi.org/10.1128/JB.00091-11
Y Sun W Zhang J Ma H Pang H Wang 2017 Overproduction of alpha-lipoic acid by gene manipulated Escherichia coli PLoS ONE 12 1 e0169369 https://doi.org/10.1371/journal.pone.0169369
Y Moriyama A Iwamoto H Hanada M Maeda M Futai 1991 One-step purification of Escherichia coli H(+)-ATPase (F0F1) and its reconstitution into liposomes with neurotransmitter transporters J Biol Chem 266 33 22141 22146 https://doi.org/10.1016/S0021-9258(18)54545-2
S Krauss C Zhang BB Lowell 2005 The mitochondrial uncoupling-protein homologues Nat Rev Mol Cell Biol 6 3 248 261 https://doi.org/10.1038/nrm1592
T Nie S Zhao L Mao Y Yang W Sun X Lin S Liu K Li Y Sun P Li 2018 The natural compound, formononetin, extracted from Astragalus membranaceus increases adipocyte thermogenesis by modulating PPARgamma activity Br J Pharmacol 175 9 1439 1450 https://doi.org/10.1111/bph.14139
S Gong H Richard JW Foster 2003 YjdE (AdiC) is the arginine: agmatine antiporter essential for arginine-dependent acid resistance in Escherichia coli J Bacteriol 185 4402 4409 https://doi.org/10.1128/JB.185.15.4402-4409.2003
RP Maharjan T Ferenci 2003 Global metabolite analysis: the influence of extraction methodology on metabolome profiles of Escherichia coli Anal Biochem 313 1 145 154 https://doi.org/10.1016/s0003-2697(02)00536-5
DR Lasko DI Wang 1993 In situ fermentation monitoring with recombinant firefly luciferase Biotechnol Bioeng 42 1 30 36 https://doi.org/10.1002/bit.260420105
J Losano D Angrimani A Dalmazzo BR Rui MM Brito CM Mendes G Kawai CI Vannucchi M Assumpcao VH Barnabe 2017 Effect of mitochondrial uncoupling and glycolysis inhibition on ram sperm functionality Reprod Domest Anim Zuchthygiene 52 2 289 297 https://doi.org/10.1111/rda.12901
T Hoang MD Smith M Jelokhani-Niaraki 2012 Toward understanding the mechanism of ion transport activity of neuronal uncoupling proteins UCP2, UCP4, and UCP5 Biochemistry 51 19 4004 4014 https://doi.org/10.1021/bi3003378
T Suzuki T Unemoto H Kobayashi 1988 Novel streptococcal mutants defective in the regulation of H+-ATPase biosynthesis and in F0 complex J Biol Chem 263 11840 11843 https://doi.org/10.1016/S0021-9258(18)37862-1
R Pandey NO Vischer JP Smelt JW Beilen van A Beek Ter WH Vos De S Brul EM Manders 2016 Intracellular pH response to weak acid stress in individual vegetative Bacillus subtilis cells Appl Environ Microbiol 82 6463 6471 https://doi.org/10.1128/AEM.02063-16
RL Golda-VanEeckhoutte LT Roof JA Needoba TD Peterson 2018 Determination of intracellular pH in phytoplankton using the fluorescent probe, SNARF, with detection by fluorescence spectroscopy J Microbiol Methods 152 109 118 https://doi.org/10.1016/j.mimet.2018.07.023
Y Sun T Fukamachi H Saito H Kobayashi 2012 Adenosine deamination increases the survival under acidic conditions in Escherichia coli J Appl Microbiol 112 4 775 781 https://doi.org/10.1111/j.1365-2672.2012.05246.x
MV Ivanova T Hoang FR McSorley G Krnac MD Smith M Jelokhani-Niaraki 2010 A comparative study on conformation and ligand binding of the neuronal uncoupling proteins Biochemistry 49 3 512 521 https://doi.org/10.1021/bi901742g
QJ Zhou J Sun YJ Zhai F Sun 2010 Prokaryotic expression of active mitochondrial uncoupling protein 1 Prog Biochem Biophys 37 1 56 62 https://doi.org/10.3724/SP.J.1206.2009.00531
W Zhang X Chen W Sun T Nie N Quanquin Y Sun 2020 Escherichia Coli increases its ATP concentration in weakly acidic environments principally through the glycolytic pathway Genes 11 9 991 https://doi.org/10.3390/genes11090991
JW Foster 2004 Escherichia coli acid resistance: tales of an amateur acidophile Nat Rev Microbiol 2 11 898 907 https://doi.org/10.1038/nrmicro1021
SY Meng GN Bennett 1992 Nucleotide sequence of the Escherichia coli cad operon: a system for neutralization of low extracellular pH J Bacteriol 174 8 2659 2669 https://doi.org/10.1128/jb.174.8.2659-2669.1992
BM Hersh FT Farooq DN Barstad DL Blankenhorn JL Slonczewski 1996 A glutamate-dependent acid resistance gene in Escherichia coli J Bacteriol 178 13 3978 3981 https://doi.org/10.1128/jb.178.13.3978-3981.1996
R Iyer C Williams C Miller 2003 Arginine-agmatine antiporter in extreme acid resistance in Escherichia coli J Bacteriol 185 22 6556 6561 https://doi.org/10.1128/JB.185.22.6556-6561.2003
A Tramonti P Visca M Canio De M Falconi D Biase De 2002 Functional characterization and regulation of gadX, a gene encoding an AraC/XylS-like transcriptional activator of the Escherichia coli glutamic acid decarboxylase system J Bacteriol 184 2603 2613 https://doi.org/10.1128/JB.184.10.2603-2613.2002
MP Castanie-Cornet JW Foster 2001 Escherichia coli acid resistance: cAMP receptor protein and a 20 bp cis-acting sequence control pH and stationary phase expression of the gadA and gadBC glutamate decarboxylase genes Microbiology 147 Pt 3 709 715 https://doi.org/10.1099/00221287-147-3-709
P Lu D Ma Y Chen Y Guo G Chen H Deng Y Shi 2013 L-glutamine provides acid resistance for Escherichia coli through enzymatic release of ammonia Cell Res 23 5 635 644 https://doi.org/10.1038/cr.2013.13
D Biase De PA Lund 2015 The Escherichia coli acid stress response and its significance for pathogenesis Adv Appl Microbiol 92 49 88 https://doi.org/10.1016/bs.aambs.2015.03.002
E Pennacchietti C D'Alonzo L Freddi A Occhialini D Biase De 2018 The glutaminase- dependent acid resistance system: qualitative and quantitative assays and analysis of its distribution in enteric bacteria Front Microbiol 9 2869 https://doi.org/10.3389/fmicb.2018.02869
Y Sun 2016 F1F0-ATPase functions under markedly acidic conditions in bacteria Advances in biochemistry in health and disease 14 Springer Cham 459 468 https://doi.org/10.1007/978-3-319-24780-9_22
BD Cain RD Simoni 1989 Proton translocation by the F1F0ATPase of Escherichia coli. Mutagenic analysis of the α subunit J Biol Chem 264 6 3292 3300 https://doi.org/10.1016/S0021-9258(18)94065-2
K Mobius R Arias-Cartin D Breckau AL Hannig K Riedmann R Biedendieck S Schroder D Becher A Magalon J Moser 2010 Heme biosynthesis is coupled to electron transport chains for energy generation Proc Natl Acad Sci USA 107 23 10436 10441 https://doi.org/10.1073/pnas.1000956107
N Kinoshita T Unemoto H Kobayashi 1984 Proton motive force is not obligatory for growth of Escherichia coli J Bacteriol 160 1074 1077 https://doi.org/10.1128/jb.160.3.1074-1077.1984
C Shibata T Ehara K Tomura K Igarashi H Kobayashi 1992 Gene structure of Enterococcus hirae (Streptococcus faecalis) F1F0-ATPase, which functions as a regulator of cytoplasmic pH J Bacteriol 174 6117 6124 https://doi.org/10.1128/jb.174.19.6117-6124.1992
F Diez-Gonzalez JB Russell 1997 Effects of carbonylcyanide-m-chlorophenylhydrazone (CCCP) and acetate on Escherichia coli O157:H7 and K-12: uncoupling versus anion accumulation FEMS Microb Lett 151 1 71 76 https://doi.org/10.1016/s0378-1097(97)00140-7
HG Yuk DL Marshall 2004 Adaptation of Escherichia coli O157:H7 to pH alters membrane lipid composition, verotoxin secretion, and resistance to simulated gastric fluid acid Appl Environ Microbiol 70 6 3500 3505 https://doi.org/10.1128/AEM.70.6.3500-3505.2004
P Shobharani PM Halami 2014 Cellular fatty acid profile and H (+)-ATPase activity to assess acid tolerance of Bacillus sp. for potential probiotic functional attributes Appl Microbiol Biotechnol 98 21 9045 9058 https://doi.org/10.1007/s00253-014-5981-3
Acknowledgements
We are grateful to Hiroshi Kobayashi (Graduate School of Pharmaceutical Sciences, Chiba University, Japan) for critical reading of this manuscript. This work was supported by the National Natural Science Foundation of China (31301019), Tianjin Synthetic Biotechnology Innovation Capacity Improvement Project (TSBICIP-KJGG-011), Pearl River S&T Nova Program of Guangzhou (201806010166) and grants by the Guangdong province of China (2014A010107024, 2017A030303065). These funding sources did not participate in study design, data collection, analysis and interpretation, or writing of the manuscript.
Author information
Authors and Affiliations
Contributions
YS designed the study and wrote the manuscript. TR, WS, JZ and ML performed the experiments. DW, JZ and YS analyzed the data. NQ and DW revised the manuscript. All authors contributed to, read, and approved the final manuscript. Correspondence to YS.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tang, R., Sun, W., Zhang, JC. et al. Expression of Human Uncoupling Protein-1 in Escherichia coli Decreases its Survival Under Extremely Acidic Conditions. Curr Microbiol 79, 77 (2022). https://doi.org/10.1007/s00284-022-02762-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00284-022-02762-3