Abstract
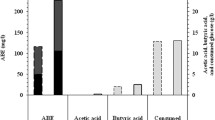
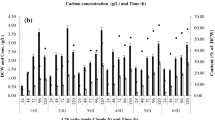
Hot springs are fascinating extreme environments for the isolation of polyextremophilic microorganisms with extraordinary characteristics. Since polyextremophilic bacterial growth are not as high as routine bacteria, the objective of this study was to investigate the effect of some environmental factors on biomass and metabolites productions in the newly isolated strain, from Larijan hot spring in Iran. The strain was identified as Aeribacillus pallidus Lhs-10 and deposited as CCUG 72355 and IBRC-M 11202 in Sweden and Iran, respectively. This thermoalkaliphilic strain can grow best at 50 °C, pH 8 and in the presence of 25 g/l NaCl. The physiological characterization of this strain show that [Ca/Mg] ratio affect its growth and biomass production with the best results obtained at the ratio of 2.5. Moreover, lactic and acetic acids production by this strain was affected by pH, aeration, and temperature, where a metabolic shift was detected from lactate to acetate production when the culture was aerated. Besides, its spores could tolerate heating at 80, 85, 90, 95 and 98 °C for 30 min without any reduction in the initial spore population, whereas D-value was defined 50 min at 98 °C. This newly lactic acid-producing strain of A. pallidus can be a promising strain that can be used in the harsh conditions in industrial processes.






Similar content being viewed by others
References
Brininger C, Spradlin S, Cobani L, Evilia C (2018) The more adaptive to change, the more likely you are to survive: protein adaptation in extremophiles. Semin Cell Dev Biol 84:158–169. https://doi.org/10.1016/j.semcdb.2017.12.016
Canganella F, Trovatelli LD (1995) Ecological and physiological studies on thermophilic bacilli from sulfataric hot springs of central Italy. J Basic Microbiol 35(1):9–19. https://doi.org/10.1002/jobm.3620350105
Chen G-Q, Jiang X-R (2018) Next generation industrial biotechnology based on extremophilic bacteria. Curr Opin Biotechnol 50:94–100. https://doi.org/10.1016/j.copbio.2017.11.016
Chen H, Ullah J, Jia J (2017) Progress in Bacillus subtilis spore surface display technology towards environment, vaccine development, and biocatalysis. J Mol Microbil Biotechnol 27(3):159–167. https://doi.org/10.1159/000475177
Connon SA, Giovannoni SJ (2002) High-throughput methods for culturing microorganisms in very-low-nutrient media yield diverse new marine isolates. Appl Environ Microbiol 68(8):3878–3885. https://doi.org/10.1128/aem.68.8.3878-3885.2002
Dominguez DC (2004) Calcium signalling in bacteria. Mol Microbiol 54(2):291–297. https://doi.org/10.1111/j.1365-2958.2004.04276.x
Donnellan JE Jr, Nags EH, Levinson HS (1964) Chemically defined synthetic media for sporulation and for germination and growth of Bacillus subtilis. J Bacteriol 87(2):332–336
Dyall-Smith ML (2006) The Halohandbook: protocols for haloarchaeal genetics. https://www.haloarchaea.com/resources/halohandbook. Accessed 18 Mar 2020
Eze UN, Okonji RE, Ibraheem O, Shonukan OO (2011) Isolation and characterization of a bacterial thermostable protease from poultry dung. Ife J Sci 13(2):289–297
Fooladi J, Sajjadian A (2010) Screening the thermophilic and hyperthermophilic bacterial population of three Iranian hot-springs to detect the thermostable α-amylase producing strain. Iran J Microbiol 2(1):46–50
Fortnagel P, Freese E (1968) Analysis of sporulation mutants. II. Mutants blocked in the citric acid cycle. J Bacteriol 95(4):1431–1438
Ghashghaei S, Etemadifar Z, Mofid MR (2018) Studies on the kinetics of antibacterial agent production in two actinomycete strains, F9 and Is5, isolated from soil samples, Iran. Iran J Sci Technol A 42(3):1139–1147. https://doi.org/10.1007/s40995-017-0171-7
Guyot JP, Calderon M, Morlon-Guyot J (2000) Effect of pH control on lactic acid fermentation of starch by Lactobacillus manihotivorans LMG 18010T. J Appl Microbiol 88(1):176–182. https://doi.org/10.1046/j.1365-2672.2000.00953.x
Horikoshi K, Antranikian G, Bull AT, Robb FT, Stetter KO (2010) Extremophiles handbook. Springer, Tokyo
Kort R, O'Brien AC, van Stokkum IHM, Oomes SJCM, Crielaard W, Hellingwerf KJ, Brul S (2005) Assessment of heat resistance of bacterial spores from food product isolates by fluorescence monitoring of dipicolinic acid release. Appl Environ Microbiol 71:3556–3564
Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35(6):1547–1549. https://doi.org/10.1093/molbev/msy096
Lane DJ (1991) 16S/23S rRNA sequencing. In: Stackebrandt E, Goodfellow M (eds) Nucleic acid techniques in bacterial systematics. Wiley, Chichester, pp 115–175
Luu-Thi H, Grauwet T, Vervoort L, Hendrickx M, Michiels CW (2014) Kinetic study of Bacillus cereus spore inactivation by high pressure high temperature treatment. Innov Food Sci Emerg Technol 26:12–17. https://doi.org/10.1016/j.ifset.2014.07.005
Ma K, Maeda T, You H, Shirai Y (2014) Open fermentative production of l-lactic acid with high optical purity by thermophilic Bacillus coagulans using excess sludge as nutrient. Bioresour Technol 151:28–35. https://doi.org/10.1016/j.biortech.2013.10.022
Olsson K, Keis S, Morgan HW, Dimroth P, Cook GM (2003) Bioenergetic properties of the thermoalkaliphilic Bacillus sp. strain TA2.A1. J Bacteriol 185(2):461–465. https://doi.org/10.1128/jb.185.2.461-465.2003
Qin J, Zhao B, Wang X, Wang L, Yu B, Ma Y, Ma C, Tang H, Sun J, Xu P (2009) Non-sterilized fermentative production of polymer-grade l-lactic acid by a newly isolated thermophilic strain Bacillus sp. 2–6. PLoS ONE 4(2):e4359–e4359. https://doi.org/10.1371/journal.pone.0004359
Rainey FA, Fritze D, Stackebrandt E (1994) The phylogenetic diversity of thermophilic members of the genus Bacillus as revealed by 16S rDNA analysis. FEMS Microbiol Lett 115(2–3):205–211. https://doi.org/10.1111/j.1574-6968.1994.tb06639.x
Rainey FA, Oren A (2006) Extremophiles. Elsevier, Academic Press
Ricca E, Cutting SM (2003) Emerging applications of bacterial spores in nanobiotechnology. J Nanobiotechnol 1(1):6–15. https://doi.org/10.1186/1477-3155-1-6
Rodrigues C, Vandenberghe LPS, Woiciechowski AL, de Oliveira J, Letti LAJ, Soccol CR (2017) Production and application of lactic acid. In: Pandey A (ed) Current developments in biotechnology and bioengineering. Elsevier, Amesterdam, pp 543–556. https://doi.org/10.1016/B978-0-444-63662-1.00024-5
Sahay H, Yadav AN, Singh AK, Singh S, Kaushik R, Saxena AK (2017) Hot springs of Indian Himalayas: potential sources of microbial diversity and thermostable hydrolytic enzymes. 3 Biotech 7(2):118. https://doi.org/10.1007/s13205-017-0762-1
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4(4):406–425. https://doi.org/10.1093/oxfordjournals.molbev.a040454
Schiraldi C, De Rosa M (2002) The production of biocatalysts and biomolecules from extremophiles. Trends Biotechnol 20(12):515–521. https://doi.org/10.1016/S0167-7799(02)02073-5
Seckbach J, Oren A, Stan-Lotter H (2013) Polyextremophiles: life under multiple forms of stress. Springer, Dordrecht
Segerer AH, Burggraf S, Fiala G, Huber G, Huber R, Pley U, Stetter KO (1993) Life in hot springs and hydrothermal vents. Origins Life Evol 23(1):77–90. https://doi.org/10.1007/bf01581992
Smith H, Brown AE (2014) Benson's microbiological applications complete version. McGraw-Hill Education, New York
Smith RJ (1995) Calcium and bacteria. In: Poole RK (ed) Advances in microbial physiology, vol 37. Academic Press, New York, pp 83–133. https://doi.org/10.1016/S0065-2911(08)60144-7
Ståhl S (1978) Calcium uptake and survival of Bacillus stearothermophilus. Arch Microbiol 119(1):17–24. https://doi.org/10.1007/bf00407922
Sudsakda SS, SrichareonPathom-Aree WW (2007) Comparison of three enrichment broths for the isolation of thermotolerant acetic acid bacteria from flowers and fruits. Res J Microbiol 2:792–795
Vos P, Garrity G, Jones D, Krieg NR, Ludwig W, Rainey FA, Schleifer KH, Whitman WB (2011) Bergey's manual of systematic bacteriology: volume 3: the Firmicutes. Springer, New York
Zebardast Roodi F, Aminzadeh S, Farrokhi N, Karkhane A, Haghbeen K (2017) Cohnella amylopullulanases: biochemical characterization of two recombinant thermophilic enzymes. PLoS ONE 12(4):e0175013. https://doi.org/10.1371/journal.pone.0175013
Acknowledgements
This study is a part of doctoral thesis that has been financially supported by the University of Isfahan, Iran and the University of Borås, Sweden. We also thank Fereshteh Javanbakht for the collection of hot spring water and soil samples.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All of the authors declare that they have no conflicts of interest in this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Harirchi, S., Etemadifar, Z., Mahboubi, A. et al. The Effect of Calcium/Magnesium Ratio on the Biomass Production of a Novel Thermoalkaliphilic Aeribacillus pallidus Strain with Highly Heat-Resistant Spores. Curr Microbiol 77, 2565–2574 (2020). https://doi.org/10.1007/s00284-020-02010-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-020-02010-6