Abstract
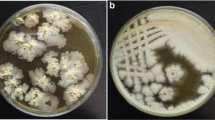
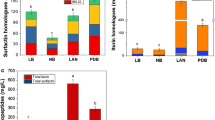
Iturin A is a very important cyclic lipopeptide produced by several B. subtilis strains and has large commercial and therapeutic application potentials but its production on industrial scale has not been realized yet. In the present study, we have observed that the strain ET-1 of Bacillus subtilis, a producer of Iturin A, can present at least three different colony morphologies, which we arbitrarily called Rough, Smooth, and Mucoid morphotypes (R-, S-, and M-form). Performing HPLC analysis, a significant difference between the amounts of Iturin A produced by the three morphotypes was found. The morphotype R-form showed the highest productivity with yields about 10 and 100 times higher than morphotypes S and M, respectively. The results show that the production of Iturin A by B. subtilis could be strongly influenced by the phenotypic heterogeneity of cells within the inoculum. Indeed, we have observed that, pasteurizing the inoculum before seeding in order to improve the homogeneity removing the phenotypes less able to synthesize the Iturin A, its yields in a bench-scale production could be significantly improved. This can represent an important control factor also at industrial scale to improve the Iturin A yields, the robustness, the replicability, and consequently the cost-effectiveness of fermentation processes.




Similar content being viewed by others
References
Ehrenberg CG (1838) Infusionsthierchen als volkommene Organismen. Leipzig
Earl AM, Losick R, Kolter R (2008) Ecology and genomics of Bacillus subtilis. Trends Microbiol 16(6):269. https://doi.org/10.1016/j.tim.2008.03.004
Zweers JC, Barák I, Becher D, Driessen AJ, Hecker M, Kontinen VP, Saller MJ, Vavrová L, van Dijl JM (2008) Towards the development of Bacillus subtilis as a cell factory for membrane proteins and protein complexes. Microb Cell Fact 7:10. https://doi.org/10.1186/1475-2859-7-10
Leclère V, BeÂcher M, Adam A, Guez JS, Wathelet B, Ongena M et al (2005) Mycosubtilin overproduction by Bacillus subtilis BBG100 enhances the organism's antagonistic and biocontrol activities. Appl Environ Microbiol 71:4577–4584. https://doi.org/10.1128/AEM.71.8.4577-4584.2005
Calvo-Garrido C, Roudet J, Aveline N, Davidou L, Dupin S, Fermaud M (2019) Microbial antagonism toward Botrytis bunch rot of grapes in multiple field tests using one Bacillus ginsengihumi strain and formulated biological control products. Front Plant Sci 10:105. https://doi.org/10.3389/fpls.2019.00105
Arrebola E, Jacobs R, Korsten L (2010) Iturin A is the principal inhibitor in the biocontrol activity of Bacillus amyloliquefaciens PPCB004 against postharvest fungal pathogens. J Appl Microbiol 108:386–395. https://doi.org/10.1111/j.1365-2672.2009.04438.x
Harwood CR, Mouillon JM, Pohl S, Arnau J (2018) Secondary metabolite production and the safety of industrially important members of the Bacillus subtilis group. FEMS Microbiol Rev 42(6):721–738. https://doi.org/10.1093/femsre/fuy028
Ambrico A, Trupo M (2017) Efficacy of cell free supernatant from Bacillus subtilis ET-1, an Iturin A producer strain, on biocontrol of green and gray mold. Postharvest Biol Technol 134:5–10. https://doi.org/10.1016/j.postharvbio.2017.08.001
Calvo H, Mendiara I, Arias E, Blanco D, Venturini ME (2019) The role of Iturin A from B. amyloliquefaciens BUZ-14 in the inhibition of the most common postharvest fruit rots. Food Microbiol 82:62–69. https://doi.org/10.1016/j.fm.2019.01.010
Dey G, Bharti R, Dhanarajan G, Das S, Dey KK, Kumar BN, Sen R, Mandal M (2015) Marine lipopeptide Iturin A inhibits Akt mediated GSK3beta and FoxO3a signaling and triggers apoptosis in breast cancer. Sci Rep 5:10316. https://doi.org/10.1038/srep10316
Zhao HB, Shao DY, Jiang CM, Shi JL, Li Q, Huang QS, Rajoka MSR, Yang H, Jin ML (2017) Biological activity of lipopeptides from Bacillus. Appl Microbiol Biotechnol 101(15):5951–5960. https://doi.org/10.1007/s00253-017-8396-0
Zhao H, Yan L, Zhang Y, Lei S, Zhao X, Shao D, Jiang C, Shi J, Sunet H (2018) Potential of Bacillus subtilis lipopeptides in anti-cancer I: induction of apoptosis and paraptosis and inhibition of autophagy in K562 cells. AMB Exp 8:78. https://doi.org/10.1186/s13568-018-0606-3
Jin H, Li K, Niu Y, Guo M, Hu C, Chen S, Huang F (2015) Continuous enhancement of Iturin A production by Bacillus subtilis with a stepwise two-stage glucose feeding strategy. BMC Biotechnol 15:53
Stein T (2005) Bacillus subtilis antibiotics: structures, syntheses and specific functions. Mol Microbiol 56:845–857. https://doi.org/10.1111/j.1365-2958.2005.04587.x
Besson F, Chevanet C, Michel G (1987) Influence of the culture medium on the production of Iturin A by Bacillus subtilis. J Gen Microbiol 133:767–772
Lin HY, Rao YK, Wu WS, Tzeng YM (2007) Ferrous ion enhanced lipopeptide antibiotic Iturin A production from Bacillus amyloliquefaciens B128. Int J Appl Sci Eng 5:123–132
Hsieh FC, Lin TC, Meng M, Kao SS (2008) Comparing methods for identifying Bacillus strains capable of producing the antifungal lipopeptide Iturin A. Curr Microbiol 56:1–5
Luo Y, Guoyi Z, Zhen Z, Xiaohui W, Wei R, Qirong S (2013) Optimization of medium composition for lipopeptide production from Bacillus subtilis N7 using response surface methodology. Korean J Microbiol Biotechnol 41:52–59
Yao D, Ji Z, Wang C, Qi G, Zhang L, Ma X, Chen S (2012) Co-producing Iturin A and poly-γ-glutamic acid from rape seed meal under solid state fermentation by the newly isolated Bacillus subtilis strain 3–10. World J Microbiol Biotechnol 28:985–991
Narendra Kumar P, Swapna TH, Khan MY, Reddy G, Hameeda B (2017) Statistical optimization of antifungal Iturin A production from Bacillus amyloliquefaciens RHNK22 using agro-industrial wastes. Saudi J Biol Sci 24:1722–1740
Batson HC (1949) Dissociation and life cycle of Bacillus subtilis. Electronic Theses and Dissertations https://openprairie.sdstate.edu/etd/1949
Kearns DB, Losick R (2005) Cell population heterogeneity during growth of Bacillus subtilis. Genes Dev 19(24):3083–3094
Fujita M, González-Pastor JE, Losick R (2005) High- and low-threshold genes in the Spo0A regulon of Bacillus subtilis. J Bacteriol 187:1357–1368
Kaern M, Elston TC, Blake WJ, Collins JJ (2005) Stochasticity in gene expression: from theories to phenotypes. Nat Rev Genet 6:451–464
Avery SV (2006) Microbial cell individuality and the underlying sources of heterogeneity. Nat Rev Microbiol 4:577–587
Berditsch M, Afonin S, Ulrich AS (2007) The ability of Aneurinibacillus migulanus (Bacillus brevis) to produce the antibiotic gramicidin S is correlated with phenotype variation. Appl Environ Microbiol 73:6620–6628
Ambrico A, Trupo M, Lopez L (2010) Identification and characterization of Bacillus subtilis ET-1. A strain with antifungal activity against fruit rot pathogens. J Plant Pathol 92(4S):72. https://doi.org/10.4454/jpp.v92i4sup.347
Ambrico A, Trupo M (2011) Evaluation of the antifungal activity of morphologically distinct colonies of Bacillus subtilis strain ET-1. J Plant Pathol 93(4S):25. https://doi.org/10.4454/jpp.v93i4.2359
Dragoš A, Lakshmanan N, Martin M, Horváth B, Maróti G, Falcón García C, Lieleg O, Kovács AT (2018) Evolution of exploitative interactions during diversification in Bacillus subtilis biofilms. FEMS Microbiol Ecol 94(1):fix155. https://doi.org/10.1093/femsec/fix155
Braun W (1947) Bacterial dissociation. A critical review of a phenomenon of bacterial variation. Bacteriol Rev 11:75–114
Pryor SW, Siebert KJ, Gibson DM, Gossett JM, Walker LP (2007) Modeling production of antifungal compounds and their role in biocontrol product inhibitory activity. J Agric Food Chem 55:9530–9536. https://doi.org/10.1021/jf0719252
Caldeira AT, Feio SS, Arteiro JM, Coelho AV, Roseiro JC (2008) Environmental dynamics of Bacillus amyloliquefaciens CCMI 1051 antifungal activity under different nitrogen patterns. J Appl Microbiol 104:808–816. https://doi.org/10.1111/j.1365-2672.2007.03601.x
Zhang Z, Ding ZT, Zhong J, Zhou JY, Shu D, Luo D, Yang J, Tan H (2017) Improvement of Iturin A production in Bacillus subtilis ZK0 by overexpression of the comA and sigA genes. Lett Appl Microbiol 64:452–458. https://doi.org/10.1111/lam.12739
Veening JW, Hamoen LW, Kuipers OP (2005) Phosphatases modulate the bistable sporulation gene expression pattern in Bacillus subtilis. Mol Microbiol 56:1481–1494. https://doi.org/10.1111/j.1365-2958.2005.04659.x
Dubnau D, Losick R (2006) Bistability in bacteria. Mol Microbiol 61:564–572. https://doi.org/10.1111/j.1365-2958.2006.05249.x
Wang JW, Zhang JJ, Yuan ZJ, Zhou TS (2007) Noise-induced switches in network systems of the genetic toggle switch. BMC Syst Biol 1(1):50
Defeu Soufo HJ (2016) A novel cell type enables B. subtilis to escape from unsuccessful sporulation in minimal medium. Front Microbiol 7:1810
Dang Y, Zhao F, Liu X, Fan X, Huang R, Gao W, Wang S, Yang C (2019) Enhanced production of antifungal lipopeptide Iturin A by Bacillus amyloliquefaciens LL3 through metabolic engineering and culture conditions optimization. Microb Cell Fact 18:68. https://doi.org/10.1186/s12934-019-1121-1
Qian S, Lu H, Meng P, Zhang C, Lv F, Bie X, Lu Z (2015) Effect of inulin on efficient production and regulatory biosynthesis of bacillomycin D in Bacillus subtilis fmbJ. Bioresour Technol 179:260–267. https://doi.org/10.1016/j.biortech.2014.11.086
Smits WK, Kuipers OP, Veening JW (2006) Phenotypic variation in bacteria: the role of feedback regulation. Nat Rev Microbiol 4:259. https://doi.org/10.1038/nrmicro1381
Čepl J, Blahůšková A, Neubauer Z, Markoš A (2016) Variations and heredity in bacterial colonies. Commun Integr Biol 9(6):e1261228. https://doi.org/10.1080/19420889.2016.1261228
Kearns DB, Chu F, Branda SS, Kolter R, Losick R (2005) A master regulator for biofilm formation by Bacillus subtilis. Mol Microbiol 55:739–749
Raj A, van Oudenaarden A (2008) Nature, nurture, or chance: stochastic gene expression and its consequences. Cell 135:216–226. https://doi.org/10.1016/j.cell.2008.09.050
Fernandes RL, Nierychlo M, Lundin L, Pedersen AE, Puentes Tellez PE, Dutta A et al (2011) Experimental methods and modeling techniques for description of cell population heterogeneity. Biotechnol Adv 29:575–599. https://doi.org/10.1016/j.biotechadv.2011.03.007
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ambrico, A., Trupo, M. & Magarelli, R.A. Influence of Phenotypic Dissociation in Bacillus subtilis Strain ET-1 on Iturin A Production. Curr Microbiol 76, 1487–1494 (2019). https://doi.org/10.1007/s00284-019-01764-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-019-01764-y