Abstract
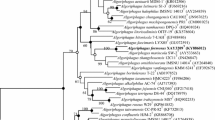
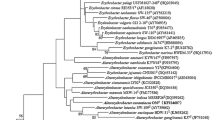
A gram-staining negative, non-motile, aerobic chemoheterotrophic, ovoid or short rod-shaped bacterium, designated as J89T, was isolated from a seawater sample collected from the coast of Yellow Sea in Qingdao, China. The strain grew at salinities of 1.0–6.0% (w/v) NaCl (optimum, 3.0%). Growth occurred at pH 6.0–9.0 (optimum, pH 7.0) and at 10–35 °C (optimum, 25–30 °C). The genomic DNA G+C content was determined to be 59.3 mol%. Q-8 was detected as the respiratory quinone. The major fatty acids (>10%) were summed feature 3 (C16:1 ω7c and/or C16:1 ω6c), summed feature 8 (C18:1 ω7c and/or C18:1 ω6c), and C16:0. The polar lipids consisted of diphosphatidylglycerol, phosphatidylglycerol, phosphatidylcholine, two unidentified phospholipids, and an unidentified polar lipid. Comparison of the 16S rRNA gene sequence indicated that the strain was most closely related (<91%) to members of the order Chromatiales in the class Gammaproteobacteria. Phylogenetic analyses showed that this strain represented a distinct phylogenetic lineage in the order Chromatiales and could not be assigned to any of the defined families in the order. On the basis of low sequence similarities and differential characteristics of strain J89T from the genera of neighboring families, the strain is proposed to be a representative of a novel genus Aquichromatium gen. nov. A new family Aquichromatiaceae with the type genus Aquichromatium is proposed. Strain J89T (=MCCC 1K03281T=CMRC C2017206T) is the type strain of the type species Aquichromatium aeriopus sp. nov.

Similar content being viewed by others
References
Arunasri K, Sasikala C, Ramana CV, Süling J, Imhoff JF (2005) Marichromatium indicum sp. nov., a novel purple sulfur gammaproteobacterium from mangrove soil of Goa, India. Int J Syst Evol Microbiol 55(2):673–679
Baek K, Choi A, Kang I, Im M, Cho J-C (2014) Granulosicoccus marinus sp. nov., isolated from Antarctic seawater, and emended description of the genus Granulosicoccus. Int J Syst Evol Microbiol 64(12):4103–4108
Biebl H, Drews G (1969) Das in vivo-Spektrum als taxonomisches Merkmal bei Untersuchungen zur Verbreitung von Athiorhodaceae. Zentralbl Bakteriol Parasitenkd Infektionskr Hyg 123(4):425–452
Bryantseva I, Gorlenko VM, Kompantseva EI, Imhoff JF, Süling J (1999) Thiorhodospira sibirica gen. nov., sp. nov., a new alkaliphilic purple sulfur bacterium from a Siberian soda lake. Int J Syst Evol Microbiol 49(2):697–703
Bryantseva I, Tourova T, Kovaleva O, Kostrikina N, Gorlenko V (2010) Ectothiorhodospira magna sp. nov., a new large alkaliphilic purple sulfur bacterium. Microbiology 79(6):780–790
Collins M, Pirouz T, Goodfellow M, Minnikin D (1977) Distribution of menaquinones in actinomycetes and corynebacteria. J Gen Microbiol 100(2):221–230
Dong X, Cai M (2001) Determinative manual for routine bacteriology. Scientific Press, Beijing (English translation)
Felsenstein J (1981) Evolutionary trees from DNA sequences: a maximum likelihood approach. J Mol Evol 17(6):368–376
Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39(4):783–791
Fitch WM (1971) Toward defining the course of evolution: minimum change for a specific tree topology. Syst Zool 20(4):406–416
Gerhardt P (1994) Methods for general and molecular bacteriology. Am Soc Microbiol, Washington DC
Gonzalez C, Gutierrez C, Ramirez C (1978) Halobacterium vallismortis sp. nov., an amylolytic and carbohydrate-metabolizing, extremely halophilic bacterium. Can J Microbiol 24(6):710–715
Hallberg KB, Hedrich S, Johnson DB (2011) Acidiferrobacter thiooxydans gen. nov., sp. nov., an acidophilic, thermo-tolerant, facultatively anaerobic iron-and sulfur-oxidizer of the family Ectothiorhodospiraceae. Extremophiles 15(2):271–279
Imhoff JF (2005) Order Chromatiales
Imhoff JF (2007) Family I. Chromatiaceae Bavendamm 1924, 125AL emend. Imhoff 1984b, 339. Bergey’s Manual® of Systematic Bacteriology: Volume 2: The Proteobacteria, Part B: The Gammaproteobacteria 2:3
Imhoff JF (2007) Family II. Ectothiorhodospiraceae Imhoff 1984b, 339VP. Bergey’s Manual® of Systematic Bacteriology: Volume 2: The Proteobacteria, Part B: The Gammaproteobacteria 2:41
Imhoff JF, Trüper HG (1977) Ectothiorhodospira halochloris sp. nov., a new extremely halophilic phototrophic bacterium containing bacteriochlorophyll b. Arch Microbiol 114(2):115–121
Imhoff JF, Süling J, Petri R (1998) Phylogenetic relationships among the Chromatiaceae, their taxonomic reclassification and description of the new genera Allochromatium, Halochromatium, Isochromatium, Marichromatium, Thiococcus, Thiohalocapsa and Thermochromatium. Int J Syst Evol Microbiol 48(4):1129–1143
Ivanova EP, Webb HK (2014) The family Granulosicoccaceae. In: the prokaryotes. Springer, pp 315-317
Kelly D (2005) Family III. Halothiobacillaceae fam. nov. Kelly and Wood 2003. Bergey’s Man Syst Bacteriol 2:58–59
Kim OS, Cho YJ, Lee K, Yoon SH, Kim M, Na H, Park SC, Jeon YS, Lee JH, Yi H (2012) Introducing EzTaxon-e: a prokaryotic 16S rRNA gene sequence database with phylotypes that represent uncultured species. Int J Syst Evol Microbiol 62(3):716–721
Kimura M (1980) A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 16(2):111–120
Kroppenstedt RM (1982) Separation of bacterial menaquinones by HPLC using reverse phase (RP18) and a silver loaded ion exchanger as stationary phases. J Liq Chromatogr 5(12):2359–2367
Kurilenko VV, Christen R, Zhukova NV, Kalinovskaya NI, Mikhailov VV, Crawford RJ, Ivanova EP (2010) Granulosicoccus coccoides sp. nov., isolated from leaves of seagrass (Zostera marina). Int J Syst Evol Microbiol 60(4):972–976
Lee K, Lee HK, Choi T-H, Kim K-M, Cho J-C (2007) Granulosicoccaceae fam. nov., to include Granulosicoccus antarcticus gen. nov., sp. nov., a non-phototrophic, obligately aerobic chemoheterotroph in the order Chromatiales, isolated from Antarctic seawater. J Microbiol Biotechnol 17(9):1483–1490
Li WJ, Xu P, Schumann P, Zhang YQ, Pukall R, Xu LH, Stackebrandt E, Jiang CL (2007) Georgenia ruanii sp. nov., a novel actinobacterium isolated from forest soil in Yunnan (China), and emended description of the genus Georgenia. Int J Syst Evol Microbiol 57(7):1424–1428
Mesbah M, Premachandran U, Whitman WB (1989) Precise measurement of the G+C content of deoxyribonucleic acid by high-performance liquid chromatography. Int J Syst Bacteriol 39(2):159–167
Minnikin D, Collins M, Goodfellow M (1979) Fatty acid and polar lipid composition in the classification of Cellulomonas, Oerskovia and related taxa. J Appl Bacteriol 47(1):87–95
Mori K, Suzuki K-i (2014) The family Thioalkalispiraceae. In: the prokaryotes. Springer, pp 653-658
Mori K, Suzuki K-i, Urabe T, Sugihara M, Tanaka K, Hamada M, Hanada S (2011) Thioprofundum hispidum sp. nov., an obligately chemolithoautotrophic sulfur-oxidizing gammaproteobacterium isolated from the hydrothermal field on Suiyo Seamount, and proposal of Thioalkalispiraceae fam. nov. in the order Chromatiales. Int J Syst Evol Microbiol 61(10):2412–2418
Mori K, Suzuki K-i, Yamaguchi K, Urabe T, Hanada S (2015) Thiogranum longum gen. nov., sp. nov., an obligately chemolithoautotrophic, sulfur-oxidizing bacterium of the family Ectothiorhodospiraceae isolated from a deep-sea hydrothermal field, and an emended description of the genus Thiohalomonas. Int J Syst Evol Microbiol 65(1):235–241
Park S, Jung Y-T, Won S-M, Park J-M, Yoon J-H (2014) Granulosicoccus undariae sp. nov., a member of the family Granulosicoccaceae isolated from a brown algae reservoir and emended description of the genus Granulosicoccus. Antonie Van Leeuwenhoek 106(5):845–852
Rijkenberg MJ, Kort R, Hellingwerf KJ (2001) Alkalispirillum mobile gen. nov., spec. nov., an alkaliphilic non-phototrophic member of the Ectothiorhodospiraceae. Arch Microbiol 175(5):369–375
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4(4):406–425
Sasser M (1990) Identification of bacteria by gas chromatography of cellular fatty acids
Schlesner H (1994) The development of media suitable for the microorganisms morphologically resembling Planctomyces spp., Pirellula spp., and other Planctomycetales from various aquatic habitats using dilute media. Syst Appl Microbiol 17(1):135–145
Sorokin DY, Lysenko AM, Mityushina LL, Tourova TP, Jones BE, Rainey FA, Robertson LA, Kuenen GJ (2001) Thioalkalimicrobium aerophilum gen. nov., sp. nov. and Thioalkalimicrobium sibericum sp. nov., and Thioalkalivibrio versutus gen. nov., sp. nov., Thioalkalivibrio nitratis sp. nov., novel and Thioalkalivibrio denitrificancs sp. nov., novel obligately alkaliphilic and obligately chemolithoautotrophic sulfur-oxidizing bacteria from soda lakes. Int J Syst Evol Microbiol 51(2):565–580
Sorokin DY, Gorlenko VM, Tsapin AI, Nealson KH, Kuenen GJ (2002) Thioalkalimicrobium cyclicum sp. nov. and Thioalkalivibrio jannaschii sp. nov., novel species of haloalkaliphilic, obligately chemolithoautotrophic sulfur-oxidizing bacteria from hypersaline alkaline Mono Lake (California). Int J Syst Evol Microbiol 52(3):913–920
Sorokin DY, Sjollema KA, Kuenen JG (2002) Thioalkalispira microaerophila gen. nov., sp. nov., a novel lithoautotrophic, sulfur-oxidizing bacterium from a soda lake. Int J Syst Evol Microbiol 52(6):2175–2182
Sorokin DY, Tourova TP, Lysenko AM, Mityushina LL, Kuenen JG (2002) Thioalkalivibrio thiocyanoxidans sp. nov. and Thioalkalivibrio paradoxus sp. nov., novel alkaliphilic, obligately autotrophic, sulfur-oxidizing bacteria capable of growth on thiocyanate, from soda lakes. Int J Syst Evol Microbiol 52(2):657–664
Sorokin DY, Tourova TP, Bezsoudnova EY, Pol A, Muyzer G (2007) Denitrification in a binary culture and thiocyanate metabolism in Thiohalophilus thiocyanoxidans gen. nov. sp. nov.–a moderately halophilic chemolithoautotrophic sulfur-oxidizing Gammaproteobacterium from hypersaline lakes. Arch Microbiol 187(6):441–450
Sorokin DY, Muntyan MS, Panteleeva AN, Muyzer G (2012) Thioalkalivibrio sulfidiphilus sp. nov., a haloalkaliphilic, sulfur-oxidizing gammaproteobacterium from alkaline habitats. Int J Syst Evol Microbiol 62(8):1884–1889
Takai K, Miyazaki M, Hirayama H, Nakagawa S, Querellou J, Godfroy A (2009) Isolation and physiological characterization of two novel, piezophilic, thermophilic chemolithoautotrophs from a deep-sea hydrothermal vent chimney. Environ Microbiol 11(8):1983–1997
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28(10):2731–2739
Venkata RV, Sasikala C, Veera VRE, Venkata RC (2010) Description of Ectothiorhodospira salini sp. nov. J Gen Appl Microbiol 56(4):313–319
Xu P, Li WJ, Tang SK, Zhang YQ, Chen GZ, Chen HH, Xu LH, Jiang CL (2005) Naxibacter alkalitolerans gen. nov., sp. nov., a novel member of the family ‘Oxalobacteraceae’ isolated from China. Int J Syst Evol Microbiol 55(3):1149–1153
Yurkov VV, Krieger S, Stackebrandt E, Beatty JT (1999) Citromicrobium bathyomarinum, a novel aerobic bacterium isolated from deep-sea hydrothermal vent plume waters that contains photosynthetic pigment-protein complexes. J Bacteriol 181(15):4517–4525
Acknowledgements
This work was supported by projects funded under the National Basic Research Program of China (2013CB955700), the National Program on Global Change and Air–Sea Interaction (GASI-03-01-02-05), the National Natural Science Foundation of China (31600399), the China Postdoctoral Science Foundation (2016M592262, 2016M602566), the Key R&D projects in Shandong Province (2015GGF01014), the Natural Science Foundation of Shandong Province of China (ZR2016CB41), Guangdong Province Higher Vocational Colleges & Schools Pearl River Scholar Funded Scheme (2014), and CNOOC Zhanjiang Branch (CNOOC-KJ 125 FZDXM 00 ZJ 001-2014).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yang, L., Tang, L., Liu, L. et al. Aquichromatium aeriopus gen. nov., sp. nov., A Non-phototrophic Aerobic Chemoheterotrophic Bacterium, and Proposal of Aquichromatiaceae fam. nov. in the Order Chromatiales . Curr Microbiol 74, 972–978 (2017). https://doi.org/10.1007/s00284-017-1275-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-017-1275-1