Abstract
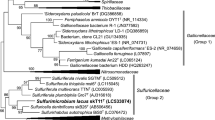
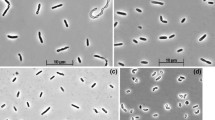
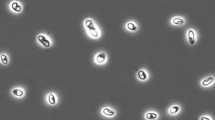
Anaerobic enrichment culture with thiocyanate as electron donor and nitrate as electron acceptor at 2 M NaCl inoculated with a mixture of sediments from hypersaline lakes in Kulunda Steppe (Altai, Russia) resulted in a selection of a binary consortium of moderately halophilic, obligately chemolithoautotrophic sulfur-oxidizing bacteria (SOB) capable of complete denitrification of nitrate with thiosulfate as the electron donor. One consortium member, strain HRhD 3sp, was isolated into pure culture with nitrate and thiosulfate using a density gradient. This strain was responsible for the reduction of nitrate to nitrite in the consortium, while a second strain, HRhD 2, isolated under microoxic conditions with thiosulfate as substrate, was capable of anaerobic growth with nitrite and thiosulfate. Nitrite, either as substrate or as product, was already toxic at very low concentrations for both strains. As a result, optimal growth under anaerobic conditions could only be achieved within the consortium. On the basis of phylogenetic analysis, both organisms were identified as new lineages within the Gammaproteobacteria. As well as thiosulfate, strain HRhD 2 can also use thiocyanate as electron donor, representing a first halophilic SOB capable of growth with thiocyanate at 2–4 M NaCl. Product and enzymatic analysis identified the “carbonyl sulfide (COS) pathway” of primary thiocyanate degradation in this new species. On the basis of phenotypic and genetic analysis, strain HRhD 2 is proposed to be assigned to a new genus and species Thiohalophilus thiocyanoxidans.




Similar content being viewed by others
References
Costa E, Pérez J, Kreft J-U (2006) Why is metabolic labour divided in nitrification? Trends Microbiol 14:213–219
Davis BJ (1964) Disk electrophoresis. Method and application to human serum proteins. Ann. N.Y.Acad.Sci 121:404–407
Derikx PJL, Op Den Camp HJM, Van Der Drift C, Van Griensven LJLD, Vogels GD (1990) Odorous sulfur compounds emitted during production of compost used as a substrate in mushroom cultivation. Appl Environ Microbiol 56:176–180
Katayama Y, Hashimoto K, Nakayama H, Mino H, Nojiri M, Ono T, Nyunoyar N, Yohda M, Takio O, Odaka M (2006) Thiocyanate hydrolase is a cobalt-containing metalloenzyme with a cysteine-sulfinic acid ligand. J Amer Chem Soc. 128:728–729
Katayama Y, Kanagawa T, Kuraishi H (1993) Emission of carbonyl sulphide by Thiobacillus thioparus grown with thiocyanate in pure and mixed cultures. FEMS Microbiol Lett 114:223–228
Katayama Y, Kuraishi H (1978) Characteristics of Thiobacillus thioparus and its thiocyanate assimilation. Can J Microbiol 24:804–810
Katayama Y, Matsushita Y, Kaneko M, Kondo M, Mizuno T, Nyunoya H (1998) Cloning of genes coding for the subunits of thiocyanate hydrolase of Thiobacillus thioparus THI 115 and their evolutionary relationships to nitrile hydratase. J Bacteriol 180:2583–2589
Katayama Y, Narahara Y, Inoue Y, Amano F, Kanagawa T, Kuraishi H (1992) A thiocyanate hydrolase of Thiobacillus thioparus. A novel enzyme catalyzing the formation of carbonyl sulphide from thiocyanate. J Biol Chem 267:9170–9175
Kelly DP (1999) Thermodynamic aspects of energy conservation by chemolithotrophic sulfur bacteria in relation to the sulfur oxidation pathways. Arch Microbiol 171:219–229
Kelly DP, Baker SC (1990) The organosulfur cycle : aerobic and anaerobic processes leading to turnover of C1-sulfur compounds. FEMS Microbiol Rev 87:241–246
Kelly DP, Stackebrandt E, Burghardt J, Wood AP (1998) Confirmation that Thiobacillus halophilus and Thiobacillus hydrothermalis are distinct species within the γ-subclass of the Proteobacteria. Arch Microbiol 170:138–140
Kelly DP, Wood AP (2000) Reclassification of some species of Thiobacillus to the newly designated genera Acidithiobacillus gen. nov., Halothiobacillus gen. nov. and Thermithiobacillus gen. nov. Int J Syst Evol Microbiol 50:511–516
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Marmur J (1961) A procedure for isolation of DNA from microorganisms. J Mol Biol 3:208–214
Murillo FM, Gugliuzzo T, Senko J, Basu P, Stolz JF (1999) A heme-C-containing enzyme complex that exhibits nitrate and nitrite reductase activity from the dissimilatory iron-reducing bacterium Geobacter metallireducens. Arch Microbiol 172:313–320
Pfennig N, Lippert KD (1966) Über das Vitamin B12–bedürfnis phototropher Schwefelbakterien. Arch Microbiol 55:245–256
Oren A (1999) Bioenergetic aspects of halophilism. Microbiol Mol Biol Rev 63:34–348
Schäfer H, Muyzer G (2001) Denaturing gradient gel electrophoresis in marine microbial ecology. Meth Microbiol 30:426–468
Sorokin DYu, Kuenen JG (2005 a) Haloalkaliphilic sulfur-oxidizing bacteria in soda lakes. FEMS Microbiol Rev 29:685–702
Sorokin DYu, Kuenen JG (2005 b) Alkaliphilic chemolithotrophs from sodas lakes. FEMS Microbiol Ecol 52:287–295
Sorokin DY, Kuenen JG, Jetten M (2001 a) Denitrification at extremely alkaline conditions in obligately autotrophic alkaliphilic sulfur-oxidizing bacterium Thioalkalivibrio denitrificans. Arch Microbiol 175:94–101
Sorokin DYu, Tourova TP, Lysenko AM, Kuenen JG (2001 b) Microbial thiocyanate utilization under highly alkaline conditions. Appl Environ Microbiol 67:528–538
Sorokin DYu, Tourova TP, Antipov AN, Muyzer G, Kuenen JG (2004) Anaerobic growth of the haloalkaliphilic denitrifying sulphur-oxidising bacterium Thialkalivibrio thiocyanodenitrificans sp. nov. with thiocyanate. Microbiology (UK) 150:2435–2442
Sorokin DY, Antipov AN, Kuenen JG (2003a) Complete denitrification in coculture of obligately chemolithoautotrophic haloalkaliphilic sulphur-oxidizing bacteria from a hypersaline soda lake. Arch Microbiol 180:127–133
Sorokin DY, Tourova TP, Sjollema KA, Kuenen JG (2003b) Thioalkalivibrio nitratireducens sp. nov., a nitrate-reducing member of an autotrophic denitrifying consortium from a soda lake. Int J Syst Evol Microbiol 53:1779–1783
Sorokin DYu, Tourova TP, Lysenko AM, Muyzer G (2006) Culturable diversity of halophilic sulfur-oxidizing bacteria in hypersaline habitats. Microbiology (UK) 152:3013–3023
Van de Peer Y, De Wachter R (1994) TREECON for Windows: a software package for the construction and drawing of evolutionary trees for the Microsoft Windows environment. Comput Applic Biosci 10:569–570
Wood AP, Kelly DP (1991) Isolation and characterisation of Thiobacillus halophilus sp. nov., a sulphur-oxidizing autotrophic eubacterium from a Western Australian hypersaline lake. Arch Microbiol 156:277–280
Wood AP, Kelly DP, McDonald IR, Jordan SL, Morgan TD, Khan S, Murrell JC, Borodina E (1998) A novel pink-pigmented facultative methylotroph, Methylobacterium thiocyanatum sp. nov., capable of growth on thiocyanate or cyanate as sole nitrogen sources. Arch Microbiol 169:148–158
Youatt J B (1954) Studies on the metabolism of Thiobacillus thiocyanooxidans. J Gen Microbiol 11:139–149
Zhilina TN, Zavarzin GA, Rainey FA, Pikuta EN, Osipov GA., Kostrikina NA (1997) Desulfonatronovibrio hydrogenovorans gen. nov., sp. nov., an alkaliphilic, sulfate-reducing bacterium. Int J Syst Bacteriol 47:144–149
Acknowledgements
This work was supported by NWO-RFBR grant (047.011.2004.010), RFBR (grant 04–04–48647), and by the Program on Molecular and Cell Biology RAS.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Figure.
Dialysis culture of strain HRhD 3sp grown anaerobically with thiosulfate and nitrate. The culture is contained in the 20 ml Centricon tube (1) with 10 kDa membrane (2) put inside the 1 L medium tank (3). The toxic product of nitrate reduction (nitrite) is diffusing out of (1) into (3) through (2); (4), magnetic bars (PPT 2.51 Mb)
Rights and permissions
About this article
Cite this article
Sorokin, D.Y., Tourova, T.P., Bezsoudnova, E.Y. et al. Denitrification in a binary culture and thiocyanate metabolism in Thiohalophilus thiocyanoxidans gen. nov. sp. nov. – a moderately halophilic chemolithoautotrophic sulfur-oxidizing Gammaproteobacterium from hypersaline lakes. Arch Microbiol 187, 441–450 (2007). https://doi.org/10.1007/s00203-006-0208-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-006-0208-3