Abstract
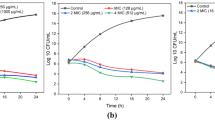
The interactions that occur between bacteria and amoebae can give through mutual relations, where both organisms benefit from the association or parasitic in which one organism benefits at the expense of the other. When these organisms share the same environment, it can result in some changes in the growth of organisms, in adaptation patterns, in morphology, development or even in their ability to synthesize proteins and other substances. In this study, the interaction between Acanthamoeba polyphaga and Staphylococcus aureus (MRSA) was evaluated using a co-culture model at different incubation times. The results showed that 89% of amoebic cells remained viable after contact with the bacteria. The bacterial isolate was visualized inside the amoeba through confocal microscopy and fluorescence for up to 216 h of co-cultivation. The lysate of amoebic culture increased the growth of S. aureus (MRSA), and the effect of supernatant of culture inhibited bacterial growth over the incubation times, suggesting that A. polyphaga produced some metabolite, that inhibited the growth of bacteria. Moreover, the encystment of the A. polyphaga was increased by the bacteria presence. The results show that A. polyphaga and S. aureus interaction may have an important influence on survival of both, and specially indicate a possible effect on the metabolics characteristics each other.







Similar content being viewed by others
References
Abul KA, Lichtman AH (2008) Imunologia Celular e Molecular. Elsevier, Rio de Janeiro
Adekambi T, Salah SB, Khlif M, Raoult D, Drancourt M (2006) Survival of environmental Mycobacteria in Acanthamoeba polyphaga. Appl Environ Microbiol 72:5974–5981
Allen PG, Dawidowicz EA (1990) Phagocytosis in Acanthamoeba. A mannose receptor is responsible for the binding and phagocytosis of yeast. J Cell Physiol 145:508–513
Anacarso I, Niederhäusern SD, Messi P, Guerrieri E, Iseppi R, Sabia C, Bondi M (2012) Acanthamoeba polyphaga, a potential environmental vector for the transmission of food-borne and opportunistic pathogens. J Basic Microbiol 52:261–268
Bowers B, Korn ED (1969) The fine structure of Acanthamoeba castellanii (Neff strain) II. J Cell Biol 41:786–805
Cateau E, Verdon J, Fernandez B, Hechard Y, Rodier MH (2011) Acanthamoeba sp: promotes the survival and growth of Acinetobacter baumanii. FEMS Microbiol Lett 319:19–25
Fieseler L, Doyscher D, Loessner MJ, Schuppler M (2014) Acanthamoeba release compounds which promote growth of Listeria monocytogenes and other bacteria. Appl Microbiol Biotechnol 98:3091–3097
Gao LY, Harb OS, Abu Kwaik Y (1997) Utilization of similar mechanisms by Legionella pneumophila to parasitize two evolutionarily distant host cells, mammalian macrophages and protozoa. Infect Immun 11:4738–4746
Greub G, Raoult D (2004) Microorganisms resistant to free-living amoebae. Clin Microbiol Rev 17:413–433
Huws SA, Morley RJ, Jones MV, Brown MRW, Smith AW (2008) Interactions of some common pathogenic bacteria with Acanthamoeba polyphaga. FEMS Microbiol Lett 282:258–265
Khan NA (2004) The pathogenesis of Acanthamoeba infections: current status and future implications. Eyetext. http://eprints.bbk.ac.uk/205/. Accessed 11 May 2016
Khan NA (2006) Acanthamoeba: biology and increasing importance in human health. FEMS Microbiol Rev 30:564–595
Koehsler M, Leitsch D, Fuernkranz U, Duchene M, Aspoeck H, Walochnik J (2008) Acanthamoeba strains lose their abilities to encyst synchronously upon prolonged axenic culture. Parasitol Res 102:1069–1072
Lamrabet O, Mba Medie F, Drancourt M (2012) Acanthamoeba polyphaga-enhanced growth of Mycobacterium smegmatis. PLoS ONE 1:298–333
Lee XY, Reimmann C, Greub G, Sufrin J, Croxatto A (2012) The Pseudomonas aeruginosa toxin l-2-amino-4-methoxy-trans-3-butenoic acid inhibits growth and induces encystment in Acanthamoeba castellanii. Microbes Infect 14:268–272
Marciano-Cabral F, Cabral G (2003) Acanthamoeba spp. as agents of disease in humans. Clin Microbiol 16:273–307
Matz C, Jurgens K (2005) High motility reduces grazing mortality of planktonic bacteria. Appl Environ Microbiol 71:921–929
Molmeret M, Jarraud S, Morin JP, Pernin P et al (2001) Different growth rates in amoeba of genotipically related environmental and clinical Legionella pneumophila strains isolated from a termal spa. Epidemiol Infect 126:231–239
Morales JL, Rivas AO, Foronda P, Martínez E, Valladares B (2005) Isolation and identification of pathogenic Acanthamoeba strains in Tenerife, Canary Islands, Spain from water sources. Parasitol Res 95:273–277
Panjwani N (2010) Pathogenesis of Acanthamoeba Keratitis. Ocul Surf 8:70–79
Schuster FL, Visvesvara GS (2004) Free-living amoebae as opportunistic and nonopportunistic pathogens of humans and animals. Int J Parasitol 34:1001–1027
Siddiqui R, Khan NA (2012) Biology and pathogenesis of Acanthamoeba. Parasites Vectors 5:6–19
Silva GJ, Souza IA, Higino JS, Siqueira-Junior JP, Pereira JV, Pereira MSV (2007) Atividade antimicrobiana do extrato de Anacardium occidentale Linn. em amostras multirresistentes de Staphylococcus aureus. Rev Bras Farmacogn 17:572–577
Thomas V, Mcdonnell G, Denyer SP, Maillard JY (2010) Free-living amoebae and their intracellular pathogenic microorganisms: risks for water quality. FEMS Microbiol Rev 34:231–259
Tomov A, Tsevtkova ED, Tomova IA, Michailova LI, Kassovski VK (1999) Persistance and multiplication of obligate anaerobe bacteria in amoebae under aerobic conditions. Anaerobe 5:19–23
Trabelsi H, Sellami A, Dendena F, Sellami H, Cheikh-rouhou F, Makni F et al (2010) Free-living amoebae (FLA): morphological and molecular identification of Acanthamoeba in dental unit water. Parasite 17:67–70
Valeru SP, Wai SN, Saeed A, Sandstrom G, Abd H (2012) ToxR of Vibrio cholera affects biofilm, rugosity and survival with Acanthamoeba castellanii. BMC Res Notes 5:33
Vanessa B, Virginie M, Nathalie Q, Marie-Hélène R, Christine I (2012) Hartmannella vermiformis can promote proliferation of Candida spp. in tap-water. Water Res 46:5707–5714
Walochnik J, Obwaller A, Aapöck H (2000) Correlations between morphological, molecular biological, and physiological characteristics in clinical and nonclinical isolates of Acanthamoeba spp. Appl Environ Microbiol 66:4408–4413
Wang X, Ahearn DG (1997) Effect of bacteria on survival and growth of Acanthamoeba castellanii. Curr Microbiol 34:212–215
Acknowledgements
The authors thank the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for financial support, the Microbiology, Immunology and Parasitology Department of the Universidade Federal do Rio Grande do Sul, Brazil.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
de Souza, T.K., Soares, S.S., Benitez, L.B. et al. Interaction Between Methicillin-Resistant Staphylococcus aureus (MRSA) and Acanthamoeba polyphaga . Curr Microbiol 74, 541–549 (2017). https://doi.org/10.1007/s00284-017-1196-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-017-1196-z