Abstract
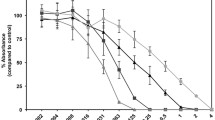
The impact of orotate accumulation in the medically important bacterium Pseudomonas aeruginosa was studied by deleting pyrE, the gene encoding orotate phosphoribosyltransferase and responsible for converting orotate into orotate monophosphate within the de novo pyrimidine synthesis pathway. The pyrE mutant accumulated orotate and exhibited decreased production of hemolysin, casein protease, and elastase. Feeding orotate at a concentration of 51.25 μM to the wild type, PAO1, likewise decreased production of these factors except for hemolysin, which was not affected. A significant increase in the pigments pyocyanin and pyoverdin was also observed. Pyocyanin increase in the pyrE mutant was heightened when the mutant was supplemented with orotate. Although pyoverdin production in the wild-type PAO1 was unaffected by orotate supplementation, a decrease in the mutant’s production was observed when supplemented with orotate. These results indicate a significant reduction in virulence factor production in the pyrE mutant and reduction in some virulence factors in the wild type when supplemented with orotate.





Similar content being viewed by others
References
Beckwith JR, Pardee AB, Austrian R, Jacob F (1962) Coordination of the synthesis of enzymes in the pyrimidine pathway of E. coli. J Mol Biol 5:618–634
Brichta DM (2003) Construction of a Pseudomonas aeruginosa dihydroorotase mutant and the discovery of a novel link between pyrimidine biosynthetic intermediates and the ability to produce virulence factors. Ph.D. Dissertation, University of North Texas
Choi KH, Schweizer HP (2005) An improved method for rapid generation of unmarked Pseudomonas aeruginosa deletion mutants. BMC Microbiol 5:30
Edmond MB, Wallace SE, McClish DK, Pfaller MA, Jones RN, Wenzel RP (1999) Nosocomial blood infection in United States hospitals: a three-year analysis. Clin Infect Dis 29:239–244
Essar DW, Eberly L, Hadero A, Crawford IP (1990) Identification and characterization of genes for a second anthranilate synthase in Pseudomonas aeruginosa: interchangeability of the two anthranilate synthases and evolutionary implications. J Bacteriol 172:884–900
Evans DR, Guy HI (2004) Mammalian pyrimidine biosynthesis: afresh insight into an ancient pathway. J Biol Chem 279:30335–33038
Kessler E, Safrin M, Olson JC, Ohman DE (1993) Secreted LasA of Pseudomonas aeruginosa is a staphylolytic protease. J Biol Chem 268:7503–7508
King EO, Ward MK, Raney DE (1954) Two simple media for the demonstration of pyocanin and fluorescin. J Lab Clin Med 44:301–307
Love WE, Bernhard JD, Bordeaux JS (2009) Topical imiquimod or fluorouracil therapy for basal and squamous cell carcinoma: asystematic review. Arch Dermatol 145:1431–1438
Maksimova NP, Blazhevich OV, Fomichev IuK (1993) The role of pyrimidines in the biosynthesis of the fluorescing pigment pyoverdin Pm in Pseudomonas putida M. Mol Gen Mikrobiol Virusol 5:22–26
Mauldin PD, Salgado CD, Hansen IS, Durup DT, Bosso JA (2009) Attributable hospital cost and length of stay associated with health care-associated infections caused by antibiotic-resistant gram-negative bacteria. Antimicrob Agents Chemother 54:109–115
Morita Y, Tomida J, Kawamura Y (2013) Responses of Pseudomonas aeruginosa to antimicrobials. Front Microbiol 4:422
Neuhard J, Kelln RA, Stauning E (1986) Cloning and structural characterization of the Salmonella typhimurium pyrC gene encoding dihydroorotase. Eur J Biochem 157:335–342
O’Donovan GA, Neuhard J (1970) Pyrimidine metabolism in microorganisms. Bacteriol Rev 34:278–343
Ornston LN, Stanier RY (1966) The conversion of catechol and protocatechuate to beta-ketoadipate by Pseudomonas aeruginosa. J Biol Chem 241:3776–3786
Pearson JP, Pesci EC, Iglewski BH (1997) Roles of Pseudomonas aeruginosa las and rhl quorum-sensing systems in control of elastase and rhamnolipid biosynthesis genes. J Bacteriol 179:5756–5767
Peters GJ, Laurensse E, Leyva A, Pinedo HM (1987) A sensitive, nonradiometric assay for dihydroorotic acid dehydrogenase using anion-exchange high-performance liquid chromatography. Anal Biochem 15:32–38
Rabaey K, Boon N, Höftee M, Verstraete W (2005) Microbial phenazine production enhances electron transfer in biofuel cells. Environ Sci Technol 39:3401–3408
Santiago MF, West TP (2002) Control of pyrimidine formation in Pseudomonas putida ATCC 17536. Can J Microbiol 48:1076–1081
Schuek KN, Breidenstein E, Hancock RE (2012) Pseudomonas aeruginosa: a persistent pathogen in cycstic fibrosis and hospital-associated infections. In: Dougherty TJ, Pucci MJ (eds) Antibiotic discovery and development, 1st edn. Springer, New York, pp 679–715
Schwartz M, Neuhard J (1975) Control of expression of the pyr genes in Salmonella typhimurium: effects of variations in uridine and cytidine nucleotide pools. J Bacteriol 121:814–822
Stintzi A, Barnes C, Xu J, Raymond KN (2000) Microbial iron transport via a siderophore shuttle: a membrane ion transport paradigm. Proc Natl Acad Sci USA 97:10691–10696
Ueda A, Attila C, Whiteley M, Wood KT (2009) Uracil influences quorum sensing and biofilm formation in Pseudomonas aeruginosa and fluorouracil is an antagonist. Microb Biotechnol 2:62–74
Wilson R, Dowling RB (1998) Lung infections. 3. Pseudomonas aeruginosa and other related species. Thorax 53:213–219
Winkler JK, Suttle DP (1988) Analysis of UMP synthase Gene and mRNA structure in hereditary orotic aciduria fibroblasts. Am J Hum Genet 43:86–94
Womack JE, O’Donovan GA (1978) Orotic acid excretion in some wild-type strains of Escherichia coli K-12. J Bacteriol 136:825–827
Acknowledgments
The authors would like to acknowledge the late Dr. Gerard A. O’Donovan for his contributions to the development of this work and Dr. Robert C. Benjamin for his insightful comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Niazy, A., Hughes, L.E. Accumulation of Pyrimidine Intermediate Orotate Decreases Virulence Factor Production in Pseudomonas aeruginosa . Curr Microbiol 71, 229–234 (2015). https://doi.org/10.1007/s00284-015-0826-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-015-0826-6