Abstract
Pyomelanin is a brown/black extracellular pigment with antioxidant and iron acquisition properties that is produced by a number of different bacteria. Production of pyomelanin in Pseudomonas aeruginosa contributes to increased resistance to oxidative stress and persistence in chronic infections. We demonstrate that pyomelanin production can be inhibited by 2-[2-nitro-4-(trifluoromethyl) benzoyl]-1,3-cyclohexanedione (NTBC). This treatment increases sensitivity of pyomelanogenic P. aeruginosa strains to oxidative stress, without altering the growth rate or resistance to aminoglycosides. As such, NTBC has potential to function as an anti-virulence factor in treating pyomelanogenic bacterial infections.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pseudomonas aeruginosa is an environmental bacterium that is capable of causing both acute and chronic infections in compromised patients. This organism is extremely adaptable, has a high level of intrinsic antibiotic resistance, a wide range of virulence factors, and the ability to form biofilms (reviewed in [1] ). Antibiotics are an essential part of treating P. aeruginosa infections, but the inherent resistance combined with emerging resistance due to selective pressure limits the therapeutic options available. As a new strategy to combat infectious disease, the specific inhibition of virulence factors has been proposed as an alternate treatment mechanism [2]. By attenuating bacterial virulence without targeting essential bacterial pathways, it may be possible to aid in the clearing of infections while minimizing selective pressures that perpetuate resistance.
Pyomelanin, a dark brown/black pigment, is a potential target for anti-virulence compounds. Pyomelanin production has been reported in P. aeruginosa isolates from urinary tract infections and chronically infected Cystic Fibrosis (CF) patients [3, 4]. Pyomelanin is one of the many forms of melanin that is produced by a wide variety of organisms. Production of pyomelanin is reported to provide a survival advantage, scavenge free radicals, bind various drugs, give resistance to light and reactive oxygen species, and is involved in iron reduction and acquisition, and extracellular electron transfer [4–9]. A number of environmental and pathogenic bacteria have been reported to produce this pigment [3, 8, 10–14]. In Shewanella oneidensis and S. algae, pyomelanin plays a role in biogeochemical cycling of metals, as pigment production enhances hydrous ferric oxide reduction and electron transfer [15–17]. In Legionella pneumophila, pigment production may contribute to pathogenesis as pyomelanin mediates ferric reduction from ferritin and transferrin [8]. Non-pyomelanogenic strains of Burkholderia cepacia are more sensitive to externally generated oxidative stress and show reduced survival in phagocytic cells [11]. In P. aeruginosa, pyomelanin production results in increased persistence and virulence in mouse infection models [3].
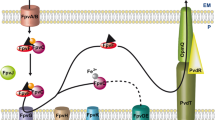
Pyomelanin is a negatively charged extracellular pigment of high molecular weight, derived from the tyrosine catabolism pathway [6, 18, 19]. 4-Hydroxyphenylpyruvate is converted to homogentisate (HGA) by 4-hydroxyphenylpyruvate dioxygenase (Hpd) (Fig. 1). HGA is then converted to 4-maleylacetoacetate by homogentisate 1,2-dioxygenase (HmgA). A loss of HmgA activity leads to the accumulation of HGA, which is secreted via the ABC transporter HatABCDE. Defects in either the ATP-binding cassette or the permease components of this transporter result in reduced pyomelanin production [4]. Once secreted from the cell, HGA auto-oxidizes and self-polymerizes to form pyomelanin. Both point mutations in hmgA and chromosomal deletions have been reported in clinical P. aeruginosa isolates producing pyomelanin [3, 10].
Hpd activity is essential for the synthesis of HGA, and ostensibly irreversible binding with 2-[2-nitro-4-(trifluoromethyl)benzoyl]-1,3-cyclohexanedione (NTBC) inhibits Hpd activity of Streptomyces avermitilis in vitro [20]. Although it was originally developed as an herbicide, NTBC is a FDA-approved treatment for type I tyrosinemia [21]. Type I tyrosinemia is the result of a defect in the tyrosine catabolism pathway, which causes the accumulation of toxic metabolites such as fumarylacetoacetate, leading to cirrhosis and cancer of the liver [22]. Binding of NTBC to Hpd prevents the accumulation of toxic metabolites and disease progression [21]. We report here on NTBC treatment of pyomelanogenic strains of P. aeruginosa, the resulting reduction in pyomelanin production, and the corresponding increase in sensitivity to oxidative stress.
Materials and Methods
Bacterial Strains and Growth Conditions
Laboratory strains of P. aeruginosa PAO1 (obtained from Carrie Harwood, University of Washington), transposon mutants hpd::tn (PW2577) and hmgA::tn (PW4489)) and the clinical isolate PA1111 were grown at 37 °C in LB supplemented with tetracycline (60 μg/ml) and gentamycin (50 μg/ml) as appropriate. The transposon mutants were obtained from the University of Washington transposon mutant collection [23]. Escherichia coli DH5α (NEB) was used as a host for recombinant plasmids, and was grown in LB with gentamycin (10 μg/ml) as appropriate.
Chemicals
NTBC (2-(2-nitro-4-trifluoromethylbenzoyl)-1,3-cyclohexanedione), H2O2, and tobramycin were purchased from Sigma-Aldrich. Gentamycin and kanamycin were purchased from Gold Bio and Fisher Scientific, respectively.
Growth Curves
Overnight cultures were grown in LB + 300 μM NTBC or LB with the corresponding amount of DMSO. The overnight cultures were diluted to OD600 0.05 in LB + 300 μM NTBC or LB + DMSO, and the optical density was measured every hour. Each sample was pelleted and resuspended in LB prior to the optical density reading to ensure that the results were not influenced by the presence of pyomelanin.
Oxidative Stress Assay
Overnight cultures were grown with NTBC (300 μM) or with a corresponding volume of DMSO as a control. Optical densities (OD600) were measured using washed cells, and cultures were diluted to equivalent OD600 values (~2.5). Tenfold serial dilutions were made in PBS containing either 300 μM NTBC or DMSO as appropriate. 5 μL of each serial dilution was spotted onto LB plates containing the indicated concentration of H2O2. Laboratory strains were incubated for 24 h and PA1111 was incubated for 45 h at 37 °C.
Determination of MICs
Minimal inhibitory concentrations (MICs) were determined by twofold serial microtiter broth dilution [24], using an inoculum of 2.75 × 105 CFU/ml. Inoculum concentration was determined using washed cells to ensure that pyomelanin production did not affect OD600 readings. NTBC was included in the appropriate wells at a final concentration of 300 μM. MICs were recorded as the lowest concentration of antibiotic inhibiting growth following 24 h of incubation at 37 °C.
Results and Discussion
Pyomelanin Production by a Clinical Isolate of P. aeruginosa
PA1111, a pyomelanogenic clinical isolate from an acute infection, was obtained from Dara Frank (Medical College of Wisconsin). This strain lacked type III secretion proteins but was cytotoxic in a tissue culture assay [25]. To determine the cause of pyomelanin production in this isolate, HmgA (PA2009) from PAO1 was expressed from pJN105 [26]. Following induction with 0.05 % and 0.1 % arabinose, pyomelanin production was eliminated in hmgA::tn and PA1111, respectively (Online resource 1a). P. aeruginosa hmgA::tn functions as a positive control for pyomelanin production as hmgA is interrupted with the ISphoA/hah transposon [23]. P. aeruginosa PAO1 and hpd::tn (isogenic to hmgA::tn) were included as negative controls; neither strain produces pyomelanin.
Since increased amounts of arabinose were required to eliminate pyomelanin production in PA1111 relative to hmgA::tn (compare 0.05–0.1 %), we assayed hpd transcript levels through RT-PCR (Online resource 1b). Quantification of the relative levels revealed that in both PAO1 and PA1111 hpd transcript was approximately 10 % more abundant than in hmgA::tn. It is unlikely that this subtle increase in hpd transcript levels is responsible for the residual pyomelanin production in PA1111 at low levels of induction (0.05 % arabinose). This suggests that the clinical isolate may have altered translation or post-translational modification resulting in increased expression or activity of Hpd.
The ability to abolish pyomelanin production in PA1111 through expression of wild-type HmgA suggested that either a chromosomal deletion or inactivation of the hmgA gene occurred, both of which have been reported in clinical isolates of P. aeruginosa [3, 10]. A third reported cause of pyomelanin production is imbalanced enzyme expression within the l-tyrosine catabolism pathway. In Vibrio cholerae (ATCC 14035), homogentisate dioxygenase and the downstream enzymes are expressed at lower levels than hydroxyphenylpyruvate dioxygenase, leading to an accumulation of HGA and pyomelanin production [27]. To determine the genetic cause of pyomelanin production in PA1111, we attempted to PCR amplify and sequence hmgA. Despite repeated attempts, we were unable to amplify hmgA via colony PCR. To verify these results, Southern hybridization was performed with DIG-labeled hmgA as a probe. No hybridization was detected between the hmgA probe and the PA1111 genome (data not shown). This, combined with our ability to complement the pyomelanin phenotype via induction of hmgA expression, suggests that a chromosomal deletion has occurred.
NTBC Inhibits Pyomelanin Production in Pseudomonas aeruginosa Without Disrupting Growth
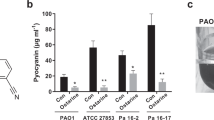
NTBC is known to bind Hpd (4-hydroxyphenylpyruvate dioxygenase) and inhibit the conversion of 4-hydroxyphenylpyruvate to homogentisate [20]. We, therefore, assayed NTBC treatment for disruption of pyomelanin production in P. aeruginosa. The two pyomelanin-producing strains (hmgA::tn and PA1111) were grown overnight with increasing amounts of NTBC. Following overnight growth, inhibition of pyomelanin production was determined visually (Fig. 2a). NTBC (300 μM) inhibited pyomelanin production in hmgA::tn, while PA1111 required higher concentrations of NTBC to inhibit pyomelanin production (900 μM). Sequencing of hpd PA1111 revealed two silent mutations upon comparison with hpd PAO1 (PA0865), demonstrating that mutations within Hpd were not responsible for the residual PA1111 pyomelanin production in the presence of 300 μM NTBC. To ensure that NTBC did not alter the growth of P. aeruginosa, we measured the optical densities of cultures grown in the presence and absence of NTBC. The laboratory strains and PA1111 grew at the same rate in the presence or absence of 300 μM NTBC (Fig. 2b), indicating that the reduction in pigmentation was not due to altered growth rates.
NTBC treatment inhibits pyomelanin production without affecting growth. a Pyomelanin production by P. aeruginosa with and without NTBC treatment. Laboratory and clinical strains were grown overnight with the indicated concentrations of NTBC. b Growth curves of laboratory and clinical strains of P. aeruginosa with and without 300 mM NTBC treatment. Strains grown without NTBC are indicated with closed symbols, while those grown with NTBC are indicated with open symbols. Wild-type PAO1 (diamonds), hpd::tn (triangles), hmgA::tn (squares), PA1111 (circles). The growth curves are compiled from three independent experiments, with error bars indicating standard error of the mean
NTBC Treatment of Pyomelanogenic Strains does not Alter Aminoglycoside MICs
It has been reported that melanin has the ability to non-specifically bind a number of diverse compounds. Isotherm analysis indicated that gentamycin had a high level of binding to synthetic melanin through a series of diverse interactions [28]. Melanin–tobramycin interactions have resulted in a decrease of antibiotic activity of 80 % under certain conditions [29]. Aminoglycosides are positively charged at physiological pH, which may contribute to the interactions with negatively charged melanin [6]. Furthermore, a significant correlation was seen between pyomelanin production in Stenotrophomonas maltophilia and resistance to specific antibiotics [14]. We therefore assayed both pyomelanin producing and non-producing strains (with and without NTBC treatment) to determine the minimal inhibitory concentrations (MICs) of aminoglycosides.
Minimal inhibitory concentrations were determined by twofold serial microtiter broth dilution [24]. Our results indicated that, under these conditions, the pyomelanin producing strains (hmgA::tn and PA1111) did not show significantly higher aminoglycoside MICs than the non-pyomelanin producing strains (PAO1 and hpd::tn, Table 1). While treatment of the pyomelanin producing strains with NTBC did inhibit pyomelanin production, the MICs remained unchanged. These data indicated that neither pyomelanin production nor NTBC treatment affect the aminoglycoside MICs for P. aeruginosa. This is in agreement with an earlier study wherein MICs were unaltered by pyomelanin production [3], and provides further clarity to the discussion within the literature regarding pyomelanin production and antibiotic resistance. Early studies of pyomelanin production reported that pyomelanogenic P. aeruginosa isolates were more sensitive to antibiotics when compared to non-pyomelanogenic strains [30]. In contrast, when Staphylococcus aureus was incubated in supernatant from pyomelanogenic P. aeruginosa, the MIC values remained unchanged [31]. When considering the results of these studies, it is critical to consider the sources of the melanin; the isotherm analysis was conducted with eumelanin (or synthetic melanin) generated from 3,4-dihydroxyphenylalanine (DOPA), not pyomelanin generated from homogentisate [6]. It is possible that the discrepancy between our results and the isotherm studies is due to the differences in melanin structures (G. Moran, personal communication). While the S. maltophilia studies did correlate pyomelanin production with increased resistance to some β-lactam antibiotics and fluoroquinolones, resistance was not detected to either gentamycin or trimethoprim/sulfamethoxazole [14]. Importantly, a direct causal relationship was not tested, and the authors acknowledged that these phenotypes could have resulted from independent mutations.
NTBC Treatment of Pyomelanin-Producing P. aeruginosa Increases Sensitivity to Oxidative Stress
The antioxidant properties of pyomelanin are proposed to contribute to the increased persistence and virulence of pyomelanogenic bacteria in infection models [3, 11, 12]. Since pyomelanogenic strains of Burkholderia cepacia and P. aeruginosa have increased resistance to hydrogen peroxide, we examined if NTBC treatment increased sensitivity of pyomelanogenic strains of P. aeruginosa to oxidative stress.
The H2O2 spot plates showed that both pyomelanogenic strains (hmgA::tn and PA1111) have increased resistance to hydrogen peroxide relative to the non-pyomelanogenic strains (PAO1 and hpd::tn) (Fig. 3). Importantly, NTBC treatment of pyomelanogenic strains resulted in increased sensitivity to 0.6 mM H2O2. This illustrates the potential use of NTBC as an anti-virulence factor. The change in sensitivity to H2O2 was smaller for PA1111 than hmgA::tn, and resulted in an approximately 24 % reduction in number of PA1111 colony forming units (based on 4 independent experiments). It is likely that the residual pyomelanin produced in PA1111 at 300 μM NTBC provides a small level of protection against oxidative stress compared to hmgA::tn. As expected, NTBC treatment of either wild-type PAO1 or hpd::tn did not affect sensitivity to H2O2.
In this report, we determined that the pyomelanin production in a strain of P. aeruginosa PA1111 isolated from an acute infection was likely due to the loss of HmgA activity resulting from a chromosomal deletion [25]. This phenotype has previously been reported for CF isolates and has been shown to decrease clearance/increase persistence in mouse models of chronic infection, suggesting that the development of pyomelanin production may confer an adaptive advantage [3, 10]. Given the antioxidant properties of pyomelanin, it is likely that pigment production would provide protection from oxidative stress in both chronic and acute infections.
This study has shown that NTBC treatment inhibited pyomelanin production by P. aeruginosa, and in doing so increased the sensitivity of both laboratory and clinical isolates to oxidative stress, as is found in the respiratory burst from macrophages and monocytes. This suggests that NTBC, as an already FDA-approved compound, has potential as an anti-virulence factor that could be used in combination with existing antibiotics. Pyomelanin is made by a wide variety of organisms, and has been reported in both chronic and acute infections. Given the number of organisms that produce pyomelanin, its functions in iron acquisition and as an antioxidant, and the presence of pyomelanin in both acute and chronic infections, there are a high number of potential applications of NTBC as an anti-virulence factor.
References
Gellatly S, Hancock R (2013) Pseudomonas aeruginosa: new insights into pathogenesis and host defenses. Pathog Dis 67:159–173. doi:10.1111/2049-632X.12033
Escaich S (2008) Antivirulence as a new antibacterial approach for chemotherapy. Curr Opin Chem Biol 12:400–408. doi:10.1016/j.cbpa.2008.06.022
Rodriguez-Rojas A, Mena A, Martin S, Borrell N, Oliver A, Blazquez J (2009) Inactivation of the hmgA gene of Pseudomonas aeruginosa leads to pyomelanin hyperproduction, stress resistance and increased persistence in chronic lung infection. Microbiology 155:1050–1057
Hunter R, Newman D (2010) A putative ABC transporter, HatABCDE, in among molecular determinants of pyomelanin production in Pseudomonas aeruginosa. J Bacteriol 192:5962–5971
Agodi A, Stefani S, Corsaro C, Campanile F, Gribaldo S, Sichel G (1996) Study of melanic pigment of Proteus mirabilis. Res Microbiol 147:167–174
Nosanchuk J, Casadevall A (2006) Impact of melanin on microbial virulence and clinical resistance to antimicrobial compounds. Antimicrob Agents Chemother 50:3519–3528
Steinert M, Engelhard H, Flugel M, Wintermeyer E, Hacker J (1995) The Lly protein protects Legionella pneumophila from light but does not directly influence its intracellular survival in Hartmannella vermiformis. Appl Environ Microbiol 61:2428–2430
Zheng H, Chatfield C, Liles M, Cianciotto N (2013) Secreted pyomelanin of Legionella pneumophila promotes bacterial iron uptake and growth under iron-limiting conditions. Infect Immun 81:4182–4191
Wang Z, Lin B, Mostaghim A, Rubin R, Glasser E, Mittraparp-arthorn P, Thompson J, Vuddhakul V, Vora G (2013) Vibrio campbellii hmgA-mediated pyomelanization impairs quorum sensing, virulence and cellular fitness. Front Microbiol 4:379. doi:10.3389/fmicb.2013.00379
Ernst R, D’Argenio D, Ichikawa J, Bangera M, Selgrade S, Burns J, Jiatt P, McCoy K, Brittnacher M, Kas A, Spencer D, Olson M, Ramsey B, Lory S, Miller S (2003) Gene mosaicism is conserved but not unique in Pseudomonas aeruginosa isolates from the airways of young children with cystic fibrosis. Environ Microbiol 5:1341–1349
Keith K, Killip L, He P, Moran G, Valvano M (2007) Burkholderia cenocepacia C5424 produces a pigment with antioxidant properties using a homogentisate intermediate. J Bacteriol 189:9057–9065
Zughaier S, Ryley H, Jackson S (1999) A melanin pigment purified from an epidemic strain of Burkholderia cepacia attenuates monocyte respiratory burst activity by scavenging superoxide anion. Infect Immun 67:908–913
Turick C, Caccavo F Jr, Tisa L (2008) Pyomelanin is produced by Shewanella algae BrY and affected by exogenous iron. Can J Microbiol/Rev Can Microbiol 54:334–339. doi:10.1139/w08-014
Liaw S-J, Lee Y-L, Hsueh P-R (2010) Multidrug resistance in clinical isolates of Stenotrophomonas maltophilia: roles of integrons, efflux pumps, phophoglucomutase (SpgM), and melanin and biofilm formation. Int J Antimicrob Agents 35:126–130. doi:10.1016/j.ijantimicag.2009.09.015
Turick C, Tisa L, Caccavo F Jr (2002) Melanin production and use as a soluble electron shuttle for Fe(III) oxide reduction and as a terminal electron acceptor by Shewanella algae BrY. Appl Environ Microbiol 68:2436–2444
Turick C, Caccavo F Jr, Tisa L (2003) Electron transfer from Shewanella algae BrY to hydrous ferric oxide is mediated by cell-associated melanin. FEMS Microbiol Lett 220:99–104
Turick C, Beliaev A, Zakrajsek B, Reardon C, Lowy D, Poppy T, Maloney A, Ekechukwu A (2009) The role of 4-hyroxyphenylpyruvate dioxygenase in enhancement of solid-phase electron transfer by Shewanella oneidensis MR-1. FEMS Microbiol Ecol 68:223–225
Arias-Barrau E, Olivera E, Leungo J, Fernandez C, Galan B, Garcia J, Diaz E, Minambres B (2004) The homogentisate pathway: a centrol catabolic pathway involved in the degradation of l-phenylalanine, l-tyrosine and 3-hydroxyphenylacetate in Pseudomonas putida. J Bacteriol 186:5062–5077
Turick C, Knox A, Becnel J, Ekechukwu A, Milliken C (2010) Properties and function of pyomelanin. In: Elnashar M (ed) Biopolymers In Tech, pp 449–72
Kavana M, Moran G (2003) Interaction of (4-hydroxyphenyl)pyruvate dioxygenase with the specific inhibitor 2-[2-nitro-4-(trifluoromethyl)benzoyl]-1,3-cyclohexanedione. Biochemistry 42:10238–10245. doi:10.1021/bi034658b
Holme E, Lindstedt S (1998) Tyrosinaemia type I and NTBC (2-(2-nitro-4-trifluoromethylbenzoyl)-1,3-cyclohexanedione). J Inherit Metab Dis 21:507–517
Russo P, Mitchell G, Tanguay R (2001) Tyrosinemia: a review. Pediatr Dev Pathol 4:212–221
Jacobs MA, Alwood A, Thaipisuttikul I, Spencer D, Haugen E, Ernst S, Will O, Kaul R, Raymond C, Levy R, Chun-Rong L, Guenthner D, Bovee D, Olson MV, Manoil C (2003) Comprehensive transposon mutant library of Pseudomonas aeruginosa. Proc Natl Acad Sci USA 100:14339–14344. doi:10.1073/pnas.2036282100
Lau C, Fraud S, Jones M, Peterson S, Poole K (2013) Mutational activation of the AmgRS two-component system in aminoglycoside-resistant Pseudomonas aeruginosa. Antimicrob Agents Chemother 57:2243–2251. doi:10.1128/AAC.00170-13
Roy-Burman A, Savel R, Racine S, Swanson B, Revadigar N, Fujimoto J, Sawa T, Frank D, Wiener-Kronish J (2001) Type III protein secretion is associated with death in lower respiratory and systemic Pseudomonas aeruginosa infections. J Infect Dis 183:1767–1774
Newman J, Fuqua C (1999) Broad-host-range expression vectors that carry the l-arabinose-inducible Escherichia coli araBAD promoter and the araC regulator. Gene 227:197–203
Sanchez-Amat A, Ruzafa C, Solano F (1998) Comparative tyrosine degradation in Vibrio cholerae strains. The strain ATCC 14035 as a prokaryotic melanogenic model of homogentisate-releasing cell. Comp Biochem Physiol B: Biochem Mol Biol 119:557–562
Bridelli M, Ciati A, Crippa P (2006) Binding of chemicals to melanins re-examined: adsorption of some drugs to the surface of melanin particles. Biophys Chem 119:137–145
Barza M, Baum J, Kane A (1976) Inhibition of antibiotic activity in vitro by synthetic melanin. Antimicrob Agents Chemother 10:569–570
Rozhavin M, Sologub V (1979) Comparison of the sensitivity of Pseudomonas aeruginosa cultures that synthesize melanin and other pigments to 12 antibiotics and 5-nitro-8-quinolinol. Antibiotiki 24:921–922
Rozhavin M (1978) Effect of Pseudomonas aeruginosa melanin on antibiotic activity. Antibiotiki 23:718–720
Acknowledgments
The authors thank Dara Frank and Carrie Harwood for their generous contribution of strains. We thank G. Moran and D. Stafford for helpful comments and discussion. University of Wisconsin Milwaukee Research Foundation holds patent no. 8,354,451; with claims broadly directed to treating or inhibiting the progression of infection of a microorganism in a patient by administering a 4-hydroxyphenylpyruvate dioxygenase-inhibiting compound such as 2-(2-nitro-4-trifluoromethylbenzoyl)-1,3-cyclohexanedione (NTBC). Inventors are Graham Moran and Pang He. This research was supported by the National Institutes of Health (R00-GM083147). The University of Washington P. aeruginosa transposon mutant library is supported by NIH P30 DK089507.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
M. Ketelboeter, L., Y. Potharla, V. & L. Bardy, S. NTBC Treatment of the Pyomelanogenic Pseudomonas aeruginosa Clinical Isolate PA1111 Inhibits Pigment Production and Increases Sensitivity to Oxidative Stress. Curr Microbiol 69, 343–348 (2014). https://doi.org/10.1007/s00284-014-0593-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-014-0593-9