Abstract
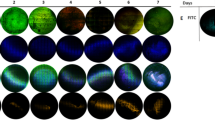
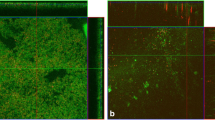
Staphylococcus epidermidis is the most frequent cause of nosocomial sepsis and catheter-related infections in which biofilm formation is considered to be one of the main virulence mechanisms. Moreover, their increased resistance to conventional antibiotic therapy enhances the need to develop new therapeutical agents. Farnesol, a natural sesquiterpenoid present in many essential oils, has been described as impairing bacterial growth. The aim of this study was to evaluate the effect of farnesol on the structure and composition of biofilm matrix of S. epidermidis. Biofilms formed in the presence of farnesol (300 μM) contained less biomass, and displayed notable changes in the composition of the biofilm matrix. Changes in the spacial structure were also verified by confocal scanning laser microscopy (CSLM). The results obtained by the quantification of extracellular polymers and by wheat germ agglutinin (WGA) fluorescent detection of glycoproteins containing β(1→4)-N-acetyl-d-glucosamine support the hypothesis that farnesol causes disruption of the cytoplasmic membrane and consequently release of cellular content.




Similar content being viewed by others
References
Al-Fattani MA, Douglas LJ (2006) Biofilm matrix of Candida albicans and Candida tropicalis: chemical composition and role in drug resistance. J Med Microbiol 55:999–1008
Cerca N, Pier GB, Oliveira R et al (2004) Comparative evaluation of coagulase-negative staphylococci (CoNS) adherence to acrylic by a static method and a parallel-plate flow dynamic method. Res Microbiol 155:755–760
Cerca N, Martins S, Sillankorva S et al (2005) Effects of growth in the presence of subinhibitory concentrations of dicloxacillin on Staphylococcus epidermidis and Staphylococcus haemolyticus biofilms. Appl Environ Microbiol 71:8677–8682
Cerca N, Jefferson KK, Oliveira R et al (2006) Comparative antibody-mediated phagocytosis of Staphylococcus epidermidis cells grown in a biofilm or in planktonic state. Infect Immun 74:4819–4855
Dubois M, Gilles KA, Hamilton JK et al (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Frølund B, Palmgren R, Keiding K et al (1996) Extraction of extracellular polymers from activated sludge using a cation exchange resin. Water Res 30:1749–1758
Gomes FIA, Teixeira P, Azeredo J et al (2009) Effect of farnesol on planktonic and biofilm cells of Staphylococcus epidermidis. Curr Microbiol 59:118–122
Gomes F, Leite B, Teixeira P et al (2011) Farnesol as antibiotics adjuvant in Staphylococcus epidermidis control in vitro. Am J Med Sci 341:191–195
Izano EA, Sadovskaya I, Vinogradov E et al (2007) Poly-N-acetylglucosamine mediates biofilm formation and antibiotic resistance in Actinobacillus pleuropneumoniae. Microb Pathog 43:1–9
Jabra-Rizk MA, Meiller TF, James CE et al (2006) Effect of farnesol on Staphylococcus aureus biofilm formation and antimicrobial susceptibility. Antimicrob Agents Chemother 50:1463–1469
Knobloch JK, Osten HV, Horstkotte MA et al (2002) Minimal attachment killing (MAK): a versatile method for susceptibility testing of attached biofilm-positive and negative Staphylococcus epidermidis. Med Microbiol Immunol 191:107–114
Kuźma Ł, Różalski M, Walencka E et al (2007) Antimicrobial activity of diterpenoids from hairy roots of Salvia sclarea L.: salvipisone as a potential anti-biofilm agent active against antibiotic resistant Staphylococci. Phytomedicine 14:31–35
Mack D, Haeder M, Siemssen N et al (1996) Association of biofilm production of coagulase-negative staphylococci with expression of a specific polysaccharide intercellular adhesin. J Infect Dis 174:881–884
Neu T, Swerhone GD, Lawrence JR (2001) Assessment of lectin-binding analysis for in situ detection of glycoconjugates in biofilm systems. Microbiology 147:299–313
Otto M (2009) Staphylococcus epidermidis—the “accidental” pathogen. Microbiology 7:555–567
Sandberg M, Määttänen A, Peltonen J et al (2008) Automating a 96-well microtitre plate model for Staphylococcus aureus biofilms: an approach to screening of natural antimicrobial compounds. Int J Antimicrob Agents 32:233–240
Sousa C, Teixeira P, Oliveira R (2009) The role of extracellular polymers on Staphylococcus epidermidis biofilm biomass and metabolic activity. J Basic Microbiol 49:363–370
Teixeira PC, Leite GM, Domingues RJ et al (2007) Antimicrobial effects of a microemulsion and a nanoemulsion on enteric and other pathogens and biofilms. Int J Food Microbiol 118:15–19
Vuong C, Kocianova S, Yao Y et al (2004) Increased colonization of indwelling medical devices by quorum-sensing mutants of Staphylococcus epidermidis in vivo. J Infect Dis 190:1498–1505
Ziebuhr W, Hennig S, Eckart M et al (2006) Nosocomial infections by Staphylococcus epidermidis: how a commensal bacterium turns into a pathogen. Int J Antimicrob Agents 28S:S14–S20
Acknowledgment
Fernanda Gomes and Pilar Teixeira fully acknowledge the financial support of Fundação para a Ciência e Tecnologia (FCT) through the grants SFRH/BD/32126/2006 and SFRH/BPD/26803/2006, respectively.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gomes, F., Teixeira, P., Cerca, N. et al. Effect of Farnesol on Structure and Composition of Staphylococcus epidermidis Biofilm Matrix. Curr Microbiol 63, 354 (2011). https://doi.org/10.1007/s00284-011-9984-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00284-011-9984-3