Abstract
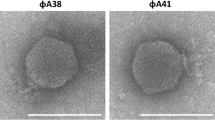
Four phages infectious to Mesorhizobium strains were identified in soil samples taken from local Robinia pseudoacacia stands. Based on their polyhedral heads and short noncontractile tails, three of the phages, Mlo30, Mam12, and Mam20, were assigned to group C of Bradley’s classification, the Podoviridae family, while phage Mlo1, with its elongated hexagonal head and a long flexible tail represented subgroup B2 bacteriophages, the Siphoviridae family. The phages were homogeneous in respect of their virulence, as they only lysed Mesorhizobium strains, but did not affect strains of Rhizobium or Bradyrhizobium. On the basis of one-step growth experiments, the average virus yield was calculated as approximately 10–25 phage particles for phages Mlo30, Mam12 and Mam20, and as many as 100–120 for phage Mlo1. The rate of phage adsorption to heat-treated cells showed differences in the nature of their receptors, which seemed to be thermal sensitive, thermal resistant, or a combination of the two. Only the receptor for phage Mlo30 was likely to be an LPS molecule, which was supported by a neutralization test. The smooth LPS with O-antigenic chains of the phage-sensitive M. loti strain completely reduced the bactericidal activity of virions at a concentration of 1 μg/ml. The molecular weights of phage DNAs estimated from restriction endonuclease cleavage patterns were in the range from ~39 kb for group C phages to ~80 kb for B2.


Similar content being viewed by others
References
Ackermann HW (2001) Frequency of morphological phage descriptions in the year 2000. Arch Virol 146:843–857
Ackermann HW, Dubow MS (1987) Viruses of prokaryotes. In: General properties of bacteriophages, vol 1. CRC Press Inc., Boca Raton
Adams MH (1959) The bacteriophages. Interscience Publishers, New York
Barnet YM (1979) Properties of Rhizobium trifolii isolates surviving exposure to specific bacteriophage. Can J Microbiol 25:979–986
Bradley DE (1967) Ultrastructure of bacteriophages and bacteriocins. Bacteriol Rev 31:230–314
Chen WX, Li GS, Qi YL, Wang ET, Li JL (1991) Rhizobium huakuii sp. nov. isolated from the root nodules of Astragalus sinicus. Int J Syst Bacteriol 41:275–280
Defives C, Werquin M, Hornez JP, Derieux JC (1993) In vivo morphogenesis and growth characteristics of phages CM (Myoviridae) virulent for Rhizobium meliloti. Curr Microbiol 27:307–310
Dhar B, Singh BD, Singh RB, Strivastava JS, Singh RM (1980) Seasonal incidence of rhizobiophages in soils around Varanasi. Indian J Exp Biol 18:1168–1170
Dhar B, Ramkrishna K, Singh BD, Singh RM (1987) Morphology and biological properties of phage RL4 of Rhizobium leguminosarum SV391. Indian J Exp Biol 25:38–41
Furuchi A, Tokunaga T (1972) Nature of the receptor substance of Mycobacterium smegmatis for D4 bacteriophage adsorption. J Bacteriol 111:404–411
Jarvis BDW, Van Berkum P, Chen WX, Nour SM, Fernandez MP, Cleyet-Marel JC, Gillis M (1997) Transfer of Rhizobium loti, Rhizobium huakuii, Rhizobium ciceri, Rhizobium mediterraneum, Rhizobium tianshanense to Mesorhizobium gen. nov. Int J Syst Bacteriol 47:895–898
Kowalski M (1967) Transduction in Rhizobium meliloti. Acta Microbiol Pol 16:7–12
Lotz W, Mayer F (1972) Electron microscopical characterisation of newly isolated Rhizobium lupini bacteriophages. Can J Microbiol 18:1271–1274
Małek W, Sajnaga E, Wdowiak-Wróbel S, Studzińska B, Święcicka I, Nosalewicz I, Słomka M, Tatara A, Gawron A (2005) Characterization of phages virulent for Sarothamnus scoparius bradyrhizobia. Curr Microbiol 51:244–249
Małek W, Wdowiak-Wróbel S, Bartosik M, Konopa G, Narajczyk M (2009) Characterization of phages virulent for Robinia pseudoacacia rhizobia. Curr Microbiol 59:187–192
Maniatis T, Fritsch EF, Sambrook J (1982) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory, New York
Martinez-Romero E, Segovia L, Mercante FM, Franco AA, Graham P, Pardo MA (1991) Rhizobium tropici, a novel species nodulating Phaseolus vulgaris L. beans and Leucaena sp. trees. Int J Syst Bacteriol 41:417–426
Mendum TA, Clark IM, Hirsch PR (2001) Characterization of two novel Rhizobium leguminosarum bacteriophages from a field release site of genetically-modified rhizobia. Antonie van Leeuwenhoek 79:189–197
Mierzwa B, Wdowiak-Wróbel S, Małek W (2009) Phenotypic, genotypic and phylogenetic characteristics of rhizobia isolated from root nodules of Robinia pseudoacacia (black locust) growing in Poland and Japan. Arch Microbiol 191:697–710
Nour SM, Fernandez MP, Normand P, Cleyet-Marel JC (1994) Rhizobium ciceri sp. nov. consisting of strains that nodulate chickpeas (Cicer arietinum L.). Int J Syst Bacteriol 44:511–522
Nuswantara S, Fujie M, Sukiman HI, Yamashita M, Yamada T, Murooka Y (1997) Phylogeny of bacterial symbionts of the leguminous tree Acacia mangium. J Ferment Bioeng 84:511–518
Russa R, Urbanik-Sypniewska T, Lindström K, Mayer H (1995) Chemical characterisation of two lipopolysaccharide species isolated from Rhizobium loti NZP2213. Arch Microbiol 163:345–351
Staniewski R, Kowalski M (1965) Effect of lysogenization on variability of phage type in Rhizobium meliloti. Acta Microbiol Pol 14:231–236
Turska-Szewczuk A, Russa R (2000) A new Mesorhizobium loti HAMBI 1129 phage isolated from Polish soil. Curr Microbiol 40:341–343
Turska-Szewczuk A, Pietras H, Borucki W, Russa R (2008) Alteration of O-specific polysaccharide structure of symbiotically defective Mesorhizobium loti mutant 2213.1 derived from strain NZP2213. Acta Biochim Pol 55:191–199
Ulrich A, Zaspel I (2000) Phylogenetic diversity of rhizobial strains nodulating Robinia pseudoacacia L. Microbiology 146:2997–3005
Vincent M (1970) A manual for the practical study of root-nodule bacteria. International biological programme, handbook no. 15. Blackwell, Oxford, Edinburgh
Wang ET, van Berkum P, Sui XH, Beyene D, Chen WX, Martinez-Romero E (1999) Diversity of rhizobia associated with Amorpha fruticosa isolated from Chinese soils and description of Mesorhizobium amorphae sp. nov. Int J Syst Bacteriol 49:51–65
Wdowiak S, Małek W, Grządka M (2000) Morphology and general characteristics of phages for Astragalus cicer rhizobia. Curr Microbiol 40:110–113
Werquin M, Ackermann HW, Levesque RC (1988) A study of 33 bacteriophages of Rhizobium meliloti. Appl Environ Microbiol 1:188–196
Werquin M, Ackermann HW, Levesque RC (1989) Characteristics and comparative study of five Rhizobium meliloti bacteriophages. Curr Microbiol 18:307–311
Yanisch-Perron C, Vieira J, Messing J (1985) Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33:103–119
Zahran HH (2001) Rhizobia from wild legumes: diversity, taxonomy, ecology, nitrogen fixation and biotechnology. J Biotechnol 91:143–153
Zając E, Russa R, Lorkiewicz Z (1975) Lipopolysaccharide as receptor for Rhizobium phage 1P. J Gen Microbiol 90:365–367
Acknowledgments
The authors wish to thank Prof. K. Lindström (Department of Applied Chemistry and Microbiology, University of Helsinki, Finland) for M. loti and M. mediterraneum strains and Prof. W.X. Chen (Department of Microbiology, College of Biology, Beijing Agricultural University, Beijing, People’s Republic of China) for M. huakuii strains. This study was supported by a grant from the Rector of M. Curie-Sklodowska University, Poland.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Turska-Szewczuk, A., Pietras, H., Pawelec, J. et al. Morphology and General Characteristics of Bacteriophages Infectious to Robinia pseudoacacia Mesorhizobia. Curr Microbiol 61, 315–321 (2010). https://doi.org/10.1007/s00284-010-9613-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-010-9613-6