Abstract
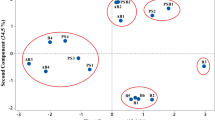
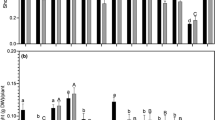
In vitro experiments had been conducted to assess the effect of pH on different native isolates of Rhizobium from the lower Brahmaputra valley region of Assam, India. The growth rate of all the Rhizobium isolates were compared growing at three different pH levels viz. 4.5, 5.5 and 6.5. All the slow-growing Rhizobium isolates (AR1, BR5, BR8, BR12, GR13, AR10, BR13, GM16, GR21) were showed better growth at all the pH levels, whereas three fast-growing Rhizobium isolates (PR7, PR12 and PR16) failed to grow at pH 4.5 and 5.5 and could show growth only at pH 6.5. Experiments were also conducted in vitro to determine the symbiotic effectiveness of all the Rhizobium isolates with test legume, green gram (Phaseolus aureus Roxb.) at three different pH levels for 42 days and significant differences were observed on nodulation, nodule dry weight and nitrogenase activity. The acid-tolerant isolates could be used as bioinoculant for enhancement of growth of leguminous plants in the acid soils.


Similar content being viewed by others
References
Carter JM, Gardner WK, Gibson AH (1994) Improved growth and yield of faba beans (Vicia faba cv. Fiord) by inoculation with strains of Rhizobium leguminosarum biovar viciae in acid soils in south-west Victoria. Aust J Agric Res 45:613–623
Chanway CP, Hynes RK, Nelson LM (1989) Plant growth promoting rhizobacteria: effects on growth and nitrogen fixation of lentil (Lens esculenta Moench) and Pea (Pisum sativum L.). Soil Biol Biochem 21:511–517
Dikshit HK, Gupta S, Gupta SR, Singh RA (2001) Variability and its characterization in Indian collections of blackgram [Vigna mungo (L.) Hepper]. PGR Newsletter 127:20–24
Graham PH, Viteri SE, Mackie F, Vargas AT, Palacios A (1982) Variation in acid soil tolerance among strains of Rhizobium phaseoli. Field Crops Res 5:121–128
Graham PH, Draeger K, Ferrey ML, Conroy MJ, Hammer BE, Martinez-Romero E, Naarons SR, Quinto C (1994) Acid pH tolerance in strains of Rhizobium and Bradyrhizobium, and initial studies on the basis for acid tolerance of Rhizobium tropici UMR1899. Can J Microbiol 40:198–207
Jones DG, Morley SJ (1981) The effect of pH on host plant preference for strains of Rhizobium trifolii using fluorescent ELISA for isolate identification. Ann. Appl. Biol 97:183–190
Kan FL, Chen ZY, Wang ET, Tian CF, Sui XH, Chen WX (2007) Characterization of symbiotic and endophytic bacteria isolated from root nodules of herbaceous legumes grown in Qinghai-tibet Plateau and in other zones of China. Arch Microbiol 188:103–115
Le CT (2003) Introductory biostatistics. Wiley, Hoboken
Liu XY, Wang ET, Li Y, Chen W (2007) Diverse bacteria isolated from root nodules of trifolium, crotalaria, mimosa grown in the subtropical regions of China. Arch Microbiol 188:1–14
Ruiz-Díez B, Fajardo S, Puertas-Mejía MA, Rosario de Felipe M, Fernández-Pascual M (2009) Stress tolerance, genetic analysis and symbiotic properties of root-nodulating bacteria isolated from Mediterranean leguminous shrubs in Central Spain. Arch Microbiol 191:35–46
Singh B, Kaur R, Singh K (2008) Characterization of Rhizobium strain isolated from the roots of Trigonella foenumgraecum (fenugreek). Afr J Biotechnol 7:3671–3676
Somasegaran P, Hoben HJ (1994) Handbook of Rhizobia. Springer, New York
Van Rossum D, Muyotcha A, De Hoop BM, Van Verseveld HW, Stouthamer AH, Boogerd FC (1994) Soil acidity in relation to groundnut-Bradyrhizobium symbiotic performance. Plant Soil 163:165–175
Vance CP, Graham PH (1995) Nitrogen fixation in agriculture: application and perspectives. In: Tikhonovich IA et al (eds) Nitrogen fixation: fundamentals and applications. Kluwer, Dordrecht, pp 77–86
Vincent JM (1970) A manual for practical study of root nodule bacteria, IBP handbook. Blackwell, Oxford
Watkin ELJ, O’Hara GW, Howieson JG, Glenn AR (2000) Identification of tolerance to soil acidity in inoculant strains of Rhizobium leguminosarum bv. Trifolii. Soil Biol Biochem 32:1393–1403
Acknowledgement
Dr. Bula Choudhury thanks Prof. R. N. S. Yadav, Center for Studies in Biotechnology, Dibrugarh University for his valuable advice and suggestions.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Choudhury, B., Azad, P. & Kalita, M.C. Variability in Symbiotic Effectiveness of Native Rhizobia in Acid Stress. Curr Microbiol 61, 85–91 (2010). https://doi.org/10.1007/s00284-009-9579-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-009-9579-4