Abstract
Glycoprotein 2 (GP2) is a widely distributed protein in the digestive tract, contributing to mucosal barrier maintenance, immune homeostasis, and antigen-specific immune response, while also being linked to inflammatory bowel disease (IBD) pathogenesis. This review sheds light on the extensive distribution of GP2 within the gastrointestinal tract and its intricate interplay with the immune system. Furthermore, the significance of GP2 autoantibodies in diagnosing and categorizing IBD is underscored, alongside the promising therapeutic avenues for modulating GP2 to regulate immunity and maintain mucosal balance.
Similar content being viewed by others
Introduction
The gastrointestinal tract, the largest immune organ in the body, is crucial for the achievement and maintenance of immune homeostasis. The mucosal immune system (MIS) serves as a critical component in regulating both innate and adaptive immune responses, as it is constantly exposed to external dietary antigens and various beneficial and harmful microorganisms. Within this well-armed MIS, multiple anti-bacterial peptides are produced by enterocytes, goblet cells, Paneth cells, and plasma cells (e.g., Ly6/PLAUR domain containing 8 (Lypd8) [1], regenerating islet-derived 3-γ (Reg3γ) [2], mucin (3), α-defensins [3, 4], lysozyme C [5], phospholipases [6], C-type lectin [7], and immunoglobulin A (IgA) [8]).
In addition to the individual importance of the gut as a digestive and immunological organ, it is now being recognized as a pivotal link between various organs, the so-called organ axis, garnering increasing attention in medical research [9,10,11]. Numerous studies have also revealed a close relationship between the intestinal microbiota and digestive organ network of the intestine, liver, and pancreas [10, 12, 13]. For instance, gut microbiota (e.g., Bacteroides, Clostridium, Escherichia, Egghertella, Fusobacterium, Lactobacillus, Peptococcus, Peptostreptococcus, Ruminococcus) are indispensable components of bile acid metabolism [10]. When the homeostatic condition of intestinal microbes is disturbed, certain bile acid metabolites, such as deoxycholic acid (DCA), can accumulate, causing intestinal inflammation and even inducing gastrointestinal carcinogenesis [10, 14].
The pancreas is known for its essential role in the exocrine and endocrine functions of digestive enzymes. In addition to its fundamental role in digestion and maintaining certain hormone levels [15], the pancreas also plays a crucial role in forming and participating in the MIS-based intestinal barrier system [9]. The pancreas secretes a range of anti-microbial substances that are released into the gut lumen, including Reg2, Reg3β, Reg3γ, and secretory phospholipase A2 (sPLA2) [9, 12, 16, 17]. Furthermore, some enzymes (e.g., trypsin and lipase) derived from the pancreas indirectly protect the homeostasis of the gut microbiota [18, 19]. Pancreatic-derived trypsin plays a crucial role in activating other anti-bacterial proteins, such as Reg3α/γ and α-defensin, within the intestine [18, 20]. Pancreatic lipase indirectly inhibits potential intestinal inflammation and protects the intestinal barrier by increasing the abundance of probiotics, such as Akkermansia muciniphila and Lactobacillus reuteri [19]. Our recent research revealed the immunological importance of the pancreatic-gut axis for GP2-mediated intestinal balance between immunity and inflammation [12]. GP2 is abundantly secreted by the pancreas and plays a frontline protective role in preventing invasion of pathogenic bacteria into the host at the gut mucosal surface [12]. These studies further solidify the significant role of the pancreas as a frontline defender and a critical component of the MIS for maintaining healthy intestinal equalization by controlling immunity and inflammation.
GP2, initially discovered in the pancreas by MacDonald and Ronzio, is a member of the zona pellucida (ZP) domain protein family [21]. Its expression has been observed in certain multipotent pancreatic progenitor cells and pancreatic acinar cells [22, 23]. In acinar cells, GP2 is co-located on the glycolipid-enriched ectoleaflet (luminal surface) in apical secretory compartments, including secretory granules [24]. Within pancreatic acinar cells, GP2 is recognized as the predominant glycoprotein within zymogen granules (ZGs), comprising 25–40% of ZG membrane (ZGM)-associated glycoproteins [24, 25]. Over the years, extensive research has confirmed that GP2 is a ubiquitous glycoprotein in the digestive tract [12]. In addition to the pancreas, our previous studies have also demonstrated the expression of GP2 on professional antigen sampling cells known as microfold cells (M cells), which are located in the follicle-associated epithelium (FAE) of Peyer’s patches, a family member of MIS inductive tissues involved in the initiation of antigen-specific mucosal immunity [26, 27]. Notably, our study visually demonstrated the continuous presence of GP2 in the intestinal lumen (e.g., duodenum, jejunum, ileum, and colon) [12]. In the colon, we observed a pronounced co-localization of GP2 with commensal bacteria and the mucus layer. This layered distribution of GP2 adds an additional physicochemical barrier to the intestinal tract. However, in the small intestine (duodenum, jejunum, and ileum), since we did not stain for mucin, whether these GP2 proteins co-localize with the mucous layer as in the colon or are merely components of the intestinal contents or feces, requires further experiments to confirm.
Molecular characteristics of GP2
According to the UniProt database (https://www.uniprot.org), GP2 is widely distributed among species, being found in mammals (e.g., humans, mice, rats, dogs, pigs, and bovines), poultry (e.g., ducks and geese), and even some fish (e.g., Danio rerio, Salmo salar, and Carassius auratus); this widespread distribution suggests the importance of conservation of GP2 across different animal species. In addition to this wide distribution, GP2 also has numerous isoforms [28, 29]. In Homo sapiens pancreas, four isoforms of GP2 have been identified and are believed to result from alternative splicing events [28, 29]. The four subtypes of GP2 exhibit pairwise similarities, with isoforms 1 and 2 sharing similarities, as do isoforms 3 and 4 [30, 31].
GP2 isoform 1 is the longest and most complete of all four isoforms that consists of 537 amino acids and several distinct domains. It begins with a signal peptide, followed by a unique long domain consisting of 197 amino acids, which contains three N-glycosylation sites, an epidermal growth factor (EGF)-like domain, and a zona pellucida (ZP) domain with three N-glycosylation sites (NCBI: NP_001007241.2, and predicted by SMART: http://smart.embl-heidelberg.de, and DTU Health Tech: https://services.healthtech.dtu.dk/) (Fig. 1). Finally, the protein has a C-terminal transmembrane domain that is removed in the Golgi apparatus upon attachment of the glycosylphosphatidylinositol inositol (GPI) anchor to the protein [30]. Among these domains, the EGF-like domain is recognized as a protein-protein binding domain [32]. The EGF-like domain is found in several ZP molecules, including ZP1, ZP2, ZP3, transforming growth factor receptor type 3 (TβRIII), and the urinary homologue of GP2, Tamm-Horsfall protein (THP) [32, 33]. The existence of an EGF-like domain may contribute to a protein’s functional role or aggregation, so this domain may be responsible for the propensity of GP2 to form tetrameric complexes.
GP2 isoforms and their roles in IBD. In humans, GP2 is continuously produced by the pancreas and released into the intestine along with pancreatic juice. Human GP2 has four distinct isoforms, namely, the longer isoforms isoform 1 (537aa) and isoform 2 (534aa), as well as the shorter isoforms isoform 3 (390aa) and isoform 4 (387aa). The GP2 released into the intestine can bind to certain pathobionts, such as Escherichia coli , preventing their invasion of the mucosal tissue. In pathological conditions like IBD, the overgrowth of pathogenic bacteria can invade the mucosa, leading to inflammation, increased levels of TNF-α, and an upregulation of pancreatic GP2 production. However, simultaneously, translocated GP2-bound bacteria may act as immune enhancers, resulting in the loss of immune tolerance to GP2 in the body. This loss of tolerance leads to the production of autoantibodies against GP2, which neutralize the luminal GP2, thereby compromising GP2’s protective function
Compared with isoform 1, isoform 2 lacks amino acids N179–181, resulting in a composition of 534 amino acids (NCBI: NP_001493.2). Isoform 3 is similar to isoform 4, both of which have relatively short isomers. Isoform 3 consists of 390 amino acids and lacks amino acids between N32 and 178 compared to isoform 1 (NCBI: NP_001007242.2), whereas the shorter isoform 4 lacks amino acids between N31 and 180 (NCBI: NP_001007243.2). The absence of these amino acids also leads to variations in the number of glycosylation sites among GP2 isoforms. The larger isoforms, 1 and 2, can be glycosylated at 8 sites, whereas the shorter isoforms (3 and 4) can only be glycosylated at 5 sites because of the absence of specific amino acids (predicted by DTU Health Tech: https://services.healthtech.dtu.dk/) (Fig. 1). The function of the GP2 isoforms might thus be influenced by alterations in their glycosylation sites due to the highly glycosylated nature of this glycoprotein [34].
Biological journey of GP2 along the pancreas and intestine axis
As mentioned above, GP2 is a highly glycosylated protein comprising approximately 15% carbohydrates in dog [35] or rat [36]. This suggests that GP2 undergoes a series of significant and complex post-translational modifications (such as asparagine-linked glycosylation and intramolecular disulfide ligation [36, 37]) within the cell before entering the ZGs and being released outside the cell.
Studies utilizing [35S] methionine pulse-chase labeling have provided valuable insights into the synthesis of GP2. Rat pancreatic acinar cells initially synthesize GP2 as an approximately 73-kDa precursor glycoprotein in the rough endoplasmic reticulum [36]. Five to six N-linked “high-mannose” oligosaccharide chains are linked to the polypeptide backbone through the dolichyl phosphate pathway. Given the use of different concentrations of tunicamycin (an N-glycosylation inhibitor) to treat isolated rat pancreatic acinar cells, the molecular weight range of the immunoprecipitated products was observed to be from 61 kDa (predicted as non-glycosylated GP2) to 73 kDa (high-mannose GP2) [36]. The precursor GP2 is then transferred to the Golgi apparatus (Fig. 2). Within the trans-Golgi network, which has a slightly acidic environment maintained by the H+ adenosine triphosphatase (ATPase), globular GP2 is released from the membrane and forms tetrameric complexes. These complexes are linked to sulfated proteoglycans, which have previously been recognized as components of the luminal surface of the ZGM [38, 39].
Biosynthesis and release of GP2 in pancreatic acinar cells. After the formation of the GP2 precursor glycoprotein, the high-mannose oligosaccharide chains are linked to the polypeptide backbone through the dolichyl phosphate pathway, which occurs within the rough endoplasmic reticulum. The precursor GP2 is then transported to the Golgi apparatus, where more complex trimming of the oligosaccharide chains and “complex sugar” glycosylation modifications occur. Subsequently, the protein becomes mature and is transported to the ZGs. Together with the ZGs, it reaches the apical plasma membrane, where it is selectively released from the membrane under the dual action of leupeptin-sensitive proteases and PI-PLC. REM: rough endoplasmic reticulum; CV: condensing vacuoles, ZG: zymogen (secretory) granules; RV: recycling vesicle; GPI: glycosylphosphatidylinositol; PI-PLC: phosphatidyl inositol-specific phospholipase C
In the Golgi apparatus, N-glycosidically linked oligosaccharide chains undergo pruning, and “complex sugars” are added by Golgi glycosyltransferases. Consequently, a mature glycoprotein that weighs approximately 78–80 kDa is formed, and mature GP2 is then transferred to ZGs [36, 40,41,42] (Fig. 2).
Another study investigated GP2 synthesis in transfected canine kidney Madin-Darby Canine Kidney (MDCK) cells expressing both rat GP2 and human GP2, as well as in primary rat pancreatic cultures [43]. That study focused on the cleavage of amino acid ends during GP2 synthesis, as proteins can only be attached to glycosylphosphatidylinositol anchors after hydrolysis of these C-terminal signal peptides [44]. That group sequenced the amino acid terminus of human GP2 and identified a similar sequence at the end of GP2 in different human samples, Gly-Leu-Asp-Leu-Asp-Cys-Gly-Ala, indicating that the cleavage site of the GP2 protein occurs after Tyr38 (MERMVGSGLLWLALVSCILTQASA [predicted signal peptide cleavage site] VQRGYGNPIEASSY [potential cleavage site] GLDLDCGAPGTPE) during post-translational modification. Therefore, the cleavage site of the human GP2 signal peptide occurs after Ala24 [43]. The predicted sites aligned with the observed size changes in the molecular weight [43].
Similarly, the biosynthesis of rat GP2 involves complex processes [36]. The post-translational modification process of GP2 is completed within approximately 60 min [36]. Interestingly, GP2 undergoes further modification, leading to a smaller molecular form 2 to 4 h after synthesis [36]. This suggests that additional modification processes may occur after the initial post-translational modifications are completed.
GP2 exhibited a complex release process. Immunocytochemical observations have revealed that GP2 exists in two forms: a membrane-localized form (such as the endoplasmic reticulum, Golgi apparatus, ZGM, and plasma membrane) and a free protein form present within the interior of both ZGs and the acinar lumen [45, 46]. This dual localization indicates the journey of GP2 from its production and trafficking to its final release within the pancreatic acinar cells (Fig. 2).
The C-terminal GPI anchor of GP2 serves as the attachment point, anchoring it to the luminal leaflet of ZGM. Substantial evidence supports the notion that proteins possessing GPI structures have a greater propensity to localize to the apical plasma membrane in some polarized cells. This suggests that GP2 may travel along with the ZGs and localize at the glycolipid-enriched ectoleaflet within apical secretory compartments [41, 47, 48].
Studies using MDCK cells transfected with rat GP2 have shown that >95% of GP2 proteins are directed to the apical plasma [41, 47, 48]. Upon pancreatic secretion was stimulated such as by gastrointestinal hormones, ZGs move towards the cell membrane and undergo fusion, leading to GP2 release into the pancreatic duct (Fig. 2). This release is facilitated by the hydrolysis of the GPI anchor by endogenous phosphatidylinositol-specific phospholipase C (PI-PLC) [49,50,51,52]. However, it is important to note that the GP2-liberation since the plasma membrane is not solely dependent on phospholipase-mediated GPI-anchored cleavage. Instead, it is secondary to proteolytic hydrolysis; that is, the cleavage of GP2 from the membrane is primarily attributed to leupeptin-sensitive proteases rather than being a secondary consequence of phospholipase-mediated GPI-anchored cleavage [41]. This selective release mechanism has the significant advantage of allowing cells to selectively regulate the release of specific proteins, such as GP2, without releasing all GPI-anchored proteins simultaneously, considering the widespread existence of GPI-anchored proteins in vivo (Fig. 2). Furthermore, another study highlighted the fact that GP2, isolated from permeable ZGs, lacks the cross-reacting determinant (CRD) epitope [53]. It is widely recognized that the CRD epitope is an antigenic determinant of the inositol ring, which is exposed when GPI-anchored proteins undergo cleavage by PI-PLC [53]. Studies have also indicated that the release of GP2 is influenced by the pH conditions. GP2 cannot be secreted from the apical plasma membrane under acidic conditions [54, 55]. However, a fraction of GP2 within the ZGs may not be membrane-associated, with approximately 45% of GP2 being membrane-independent [52]. Upon cleavage and release, GP2 exhibits self-polymerizing properties, leading to the formation of macromolecules within the pancreas. These compounds are subsequently released along with digestive enzymes [51, 52].
The remarkably high concentration of GP2 in ZGs suggests its crucial role in ZGs’ function. Previous studies have also explored the significance of GP2 in ZG formation [42, 56, 57]. As discussed above, GP2 attaches to the ZGM through a GPI anchor (Fig. 2). Expanding on this understanding, Scheele et al. proposed that GP2 could form submembrane matrices with proteoglycans, potentially facilitating membrane sorting during granule assembly, ensuring stability during particle storage, and regulating zymogen transport from the apical plasma membrane following exocytosis [40].
Jacob et al. provided further support for this hypothesis by demonstrating the binding of amylase to GP2 in vitro under varying pH conditions, suggesting a potential role of GP2 in sorting aggregated secretory digestive enzymes into ZGs [58]. Colomer et al. also arrived at a similar conclusion by comparing the secretory form of GP2-GPI- GP2 with pancreatic exocrine cell AR42J and pituitary endocrine cell AtT20 [42]. In addition, Scheele et al. posited that the liberation of the GP2/proteoglycan matrix from the apical plasma membrane might be a prerequisite for the internalization and subsequent reuse of ZGM [40]. Subsequent evidence supporting this hypothesis was presented by the same research group, which suggested that the alkaline environment within the acinar lumen modulates the release of the matrix from ZGs during exocytosis, leading to further endocytosis of the granules. Activation of PI-PLC is also involved in this process [54]. Several endocytosis-related proteins, such as pp60, p62yes, and caveolin, were also found to be abundant in GPI-anchored membranes and co-immunoprecipitated with GP2, suggesting that GP2 may regulate acinar cell endocytosis through a tyrosine kinase regulation pathway [59].
However, some other viewpoints suggest that GP2 may not be necessary for ZG formation [60]. Instances of ZG formation in the absence of GP2 have been previously reported. For example, the GP2 mRNA and protein expression do not occur until birth, but a significant number of ZGs are produced during embryonic development (15–21 days) in rats [61]. Partially differentiated pancreatic acinar cell carcinoma lines, specifically AR42J [61] and Reddy cells [62], were also found to be deficient in GP2. In addition, a study observed that ZGs in GP2 knockout mice displayed a normal morphology, number, and protein content, except for the absence of GP2, as confirmed by electron microscopy [60]. Therefore, further investigations are required to elucidate the underlying mechanisms [63].
Notably, our recent study provides compelling evidence of increased pancreatic GP2 expression in individuals with colitis [12]. In a mouse model of dextran sodium sulfate (DSS)-induced colitis, pancreatic GP2 levels were elevated. GP2 increase occurs without any apparent pancreatic pathological, histological (such as inflammatory cell infiltration), or physiological changes, such as altered pancreatic lipase expression [12]. Furthermore, elevated GP2 expression is specific to pancreatic cells, with a normal GP2 expression observed in follicle-associated epithelial cells of Peyer’s patches, which are also known to express GP2 [12].
To investigate the underlying mechanism behind the increased GP2 levels without obvious pathological changes in the pancreas, we investigated the synthesis, transport, and release processes of GP2. In particular, we focused on the examination of various guanosine triphosphate (GTPases) (e.g., Ras-Related Protein Rab3b, Rab6a, Rab8a, Rab27b, and Rap1a) and vesicular trafficking proteins (e.g., vesicle-associated membrane protein [Vamp] 2, Vamp3, Vamp8, syntaxin 3, and syntaxin 7) involved in the ZG transport system [12]. We also explored the expression of several enzymes that might cleave the GPI anchor, such as trypsin 5, phospholipases (Plcxd2, Plch1), and carboxypeptidases (Cpa1 and Cpa2). Data showed that the expression of the examined indicators did not change significantly. Nevertheless, based on our observations, we suggest that in cases of colitis, the increase in luminal GP2 may be attributed to de novo synthesis [12]. Further investigations revealed that the increased expression of GP2 in the pancreas was closely correlated with tumor necrosis factor-α (TNF-α), an inflammatory agent that is markedly elevated in cases of colitis [12]. Furthermore, our study successfully demonstrated that the released GP2 protein within the gut colocalizes with mucin, forming the primary line of defense against bacterial invasion [12].
Overall, the release of GP2 from ZGs involves complex mechanisms, including GPI anchor hydrolysis, proteolytic cleavage, disease, and pH sensitivity. These processes ensure the selective release of GP2.
Multiple functions of GP2 in the digestive tract
The function of the GP2 protein within ZGs was extensively discussed in the previous section. However, considering the significant release of GP2 outside the cell after passing through the stages within ZGs, and the intricate nature of the human body, which typically avoids unnecessary processes, scientists have speculated that GP2 likely serves additional functions beyond ZGs. However, the precise role of GP2 in the pancreas and subsequent digestive tract has remained unclear [60].
Studies focused on the characterization of THP, a urinary counterpart of GP2, have provided valuable insights into the physiological functions of GP2 [53, 64]. THP is the predominant protein found in the urinary tract and originates from the renal tubular epithelial cells located in the ascending limb of Henle’s loop [53, 65]. In addition, THP has been found to regulate innate and adaptive immunity in the urinary tract [66] as well as enhanced the activity of monocyte proliferation and polymorphonuclear neutrophil phagocytosis [33, 67]. Studies have revealed that GP2 and THP share a common ancestral gene set, and divergence of genes between the urinary and digestive systems during development is the fundamental reason for their tissue specificity [68]. According to the GenBank database (https://www.ncbi.nlm.nih.gov/genbank/), the amino acid sequence of human GP2 exhibited 53% identity (percentage of positions in a pairwise alignment of 2 amino acid sequences with identical residues) and 85% similarity (percentage of positions in the alignment where the residues are either identical or have similar properties) with that of human THP [64].
Consequently, it appears that the functional characteristics of these two homologous proteins are partially preserved across both organ systems. Extensive research has been conducted on the anti-microbial function of THP in the urinary system [69, 70]. THP has been found to bind to type 1 fimbriae of Escherichia coli in the urinary tract, thereby preventing bacterial adhesion and invasion and subsequently reducing the likelihood of urinary tract infections [70,71,72].
Expression of GP2 on the apical surface of M cells located in the FAE of Peyer’s patches [26, 27], as well as its overexpression in biopsy samples taken from the colon of Crohn’s disease (CD) patients, has been extensively documented [12, 73,74,75]. In IBD pathophysiology, the intestinal microbiome plays a vital role in perpetuating inflammatory processes [76]. CD patients have been found to have a higher density of mucosal microbiota, particularly adherent bacteria interacting with Peyer’s patches, than healthy individuals [77, 78]. Building upon this observation, we made the assumption that GP2 might possess an immunobiological function that contributes to the creation of mucosal equilibrium between gut microbiota and the host, including prevention of adhesion and invasion of pathobionts. Our previous study showed that GP2 expressed on M cells was able to specifically bind to type 1 fimbrial adhesin (FimH) as the transcytotic receptor of bacteria [27]. FimH is a type 1 pili expressed in multiple pathogenic microorganisms, including E. coli and Salmonella typhimurium [79, 80]. Once M cells are anchored, GP2 attaches to the bacteria. Through trans-endocytosis, M cell-engulfed bacteria are captured by underlying dendritic cells, leading to the initiation of antigen-specific mucosal immunity [27, 73]. The absence of GP2 or FimH results in the reduction of antigen-specific serum immunoglobulin G (IgG) and gut IgA production by mucosal immunization targeting Peyer’s patches [27]. These findings suggest that GP2 is also involved in the induction and regulation of antigen-specific humoral immunity in both systemic and mucosal compartments especially expressed on Peyer’s patches. Furthermore, GP2 has been shown to enhance the E. coli phagocytic ability of monocytes [81].
As mentioned earlier, our recent study demonstrated that GP2, distributed extensively throughout the small and large intestines, is primarily derived from pancreatic acinar cells [12]. To further elucidate the immunobiological role of GP2 in vivo, pancreatic GP2-specific deficient mice were developed [12]. Following tamoxifen treatment, these mice exhibited a significant decrease in GP2 expression in the pancreas as well as in the luminal side of the digestive tract [12]. Notably, these GP2-deficient mice demonstrated heightened susceptibility to colon inflammation induced by DSS, resulting in more severe inflammatory responses than control mice [12]. We further investigated whether or not these pancreatic GP2 proteins could bind to FimH in a manner similar to anchored GP2 in M cells [12]. The pancreatic GP2 was capable of binding to FimH-positive E. coli. Furthermore, flow cytometry data demonstrated a notable increase in GP2-binding bacteria in inflamed GP2-deficient mice, which supports the crucial protective effect of GP2 [12] (Fig. 1).
Considering the interaction between GP2 and E. coli, we also explored whether GP2 might play a role in shaping the bacterial composition within the gut [12]. To investigate this notion further, we conducted 16S rRNA sequencing analysis to examine the composition of the intestinal commensal microbiota in both wild-type (WT) and GP2-deficient (GP2−/−) mice. The absence of GP2 did not appear to have a significant impact on the composition of the commensal microbiota at the phylum or genus levels. However, a closer examination revealed an increase in the populations of Helicobacter sp. and Clostridium sp., while the population of Bacteroides acidifaciens decreased in GP2−/− mice. Nevertheless, the specific influence of these microbial changes on gut homeostasis and disease remains to be thoroughly investigated [82, 83]. Furthermore, we extended our investigation to young mice at the age of 2 weeks, and once again, we did not observe any substantial effects of GP2 deficiency on the composition of the gut microbiota [12].
In addition to assessing the impact of GP2 on the diversity of gut microbial species, we explored whether GP2 deficiency might lead to an increased bacterial load in the intestinal mucosa, which is particularly relevant since elevated mucosal bacterial numbers are often associated with colitis [84]. Using quantitative PCR, we measured the sizes of the total bacterial population (Eubacteria) and several specific bacterial populations, including E. coli, Lactobacillus, Bacteroides, Helicobacter, and Proteus, in the colons of mice [12]. The results indicated that in GP2-deficient mice, there was a notable increase in the population of mucosa E. coli compared to WT mice. This finding suggests that GP2 contributes to the regulation of the mucosal microbiota in the intestine, primarily through its influence on the population of E. coli. Consequently, GP2 deficiency may exacerbate the severity of colitis, considering the well-established association between increased E. coli levels and colonic inflammation [12]. Furthermore, our in vitro experiments indicated that binding to recombinant GP2 did not appear to affect bacterial growth.
To further understand GP2 protein binding to FimH, several studies have provided evidence that the glycosylation of GP2, particularly D-mannose [34, 85] and N-65 glycosylation [70], play critical roles. Furthermore, the binding capabilities of FimH and GP2 differ among variants/isoforms [34, 85]. GP2 isoforms 1 and 2 are considered to have a stronger affinity than short isoforms 3 and 4, possibly due to the presence of more glycosylation sites in these longer isoforms, providing additional asparagine residues and corresponding mannose residues, as discussed above [85]. However, according to the report from Derer et al. isoform 4, the dominant free-wandering GP2 isoform in the intestinal tract, binds mainly to the FimH of pathogens [29]. In addition, the different FimH variants and flow conditions (to simulate peristalsis in the natural gut) within the intestine have also been reported to influence the binding ability between GP2 and FimH [85]. Indeed, GP2 appears to have more than protective roles. In certain situations, there have been reports of GP2 binding to certain bacterial toxins, leading to its pathogenic effects. Studies have found that GP2 also exhibits binding affinity towards hemagglutinin A1 (HA) of botulinum neurotoxin, causing food-borne botulism [86]. HA-translocated GP2 disrupts the adherent junctions between M cells and enterocytes, impairing the epithelial barrier function [86].
Similar to the THP protein introduced above, GP2 is involved in more than just the gut-bacterial interaction and is thus capable of regulating the MIS. A previous study indicated that GP2 is recognized as the binding partner of scavenger receptors expressed on endothelial cell I (SREC-I), which are found on not only endothelial cells but also monocyte-derived dendritic cells (DCs) [87]. Given the widespread distribution of GP2 in the digestive tract, it is possible that DCs expressing SREC-I interact with GP2 or GP2 complexes, leading to internalization of GP2 and GP2 complexes by DCs. This suggests that GP2 may have a profound effect on the immune system, considering the pivotal role of DCs in both innate and adaptive immunity [87].
GP2 has also been associated with T cells. A previous study showed that GP2 expression in both peripheral blood mononuclear cells (PBMCs) and Caco-2 epithelial cells are regulated by activated human T cells and TNF-α inhibitors [81]. Intriguingly, treatment with recombinant GP2 showed significant effects on human intestinal epithelial, mucosal, and peripheral T cells, including reduced proliferation and apoptosis. GP2 also appears to regulate cytokine secretion, leading to decreased proinflammatory TNF-α and interleukin-17 (IL-17) levels and increased regulatory transforming growth factor β1 (TGF-β1) levels in PBMCs and freshly resected mucosal specimens from healthy volunteers or patients with non-inflammatory disorders [81]. In mucosal specimens, GP2 treatment resulted in decreased secretion of proinflammatory C-X-C motif chemokine ligand 8 (CXCL8) and increased secretion of regulatory TGF-β1 [81]. In addition, GP2-stimulate-intestinal epithelial cells demonstrated strong chemoattractant properties for T cells, which produced an effect similar to that of CXCL8 [81]. Based on these findings, Werner et al. proposed that GP2 might act as an anti-inflammatory and immunosuppressive agent in the intestinal mucosal system through its interaction with regulatory T cells [81].
Anti-GP2 autoantibodies in IBD
The increased expression of GP2 in IBD has been extensively studied [12]. However, upregulation of GP2 also poses a risk of inducing autoantibodies [74]. Autoimmune responses were identified as essential triggers and perpetrators of IBD in patients with ulcerative colitis (UC) and CD, particularly in CD, as early as the 1950s and the 1970s, respectively [88]. Humoral autoimmune responses against pancreatic exocrine secretion have been observed in approximately 30% of patients with CD and 8% of patients with UC [89, 90]. It has also been noted that patients with pancreatitis are more susceptible to CD than the general population [91]. Pancreatic autoantibodies (PAB) exist in approximately 68% of CD patients with extraintestinal complications, such as idiopathic chronic pancreatitis [92,93,94]. Specific targets of PAB were identified in 2009, with GP2 being recognized as the primary autoantigen [73]. Furthermore, evidence indicates that, in addition to increased levels of pancreatic-synthesized GP2, both GP2 mRNA transcription and translation are elevated in the intestines of patients with CD [73, 74].
Two types of PAB were labeled based on the location of the indirect immunofluorescence signal [95]. The first type exhibits drop-like staining within the extracellular acinar lumen, whereas the second type shows speckled staining in the cytoplasm of acinar cells [95]. This distinction may arise from the two different states of GP2, which is released into the intestinal lumen alongside digestive enzymes and is also present in the ZGM of pancreatic acinar cells as a GPI-anchored membrane protein [52]. As mentioned above, the self-polymerization capability of GP2, resulting in the formation of high-molecular-weight structures [52], might generate new autoepitopes [96]. These factors contribute to the diversity and location specificity of PAB staining patterns.
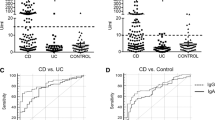
The significance of anti-GP2 autoantibodies in the serological diagnosis of IBD has been thoroughly studied [74, 95, 97]. Studies have shown that patients with CD exhibit a higher prevalence of anti-GP2 autoantibodies than patients with UC. The incidence of anti-GP2 autoantibodies ranges from 21 to 45% in CD patients, 2 to 19% in UC patients, and 1 to 8% in healthy subjects [73, 97,98,99]. Studies have also shown that GP2 levels in the feces of CD patients are elevated compared with those in healthy individuals [12, 75]. Furthermore, patients with confirmed celiac disease also exhibit an autoimmune response to GP2 [94, 99]. Therefore, anti-GP2 autoantibodies can serve as a complementary diagnostic tool for stratifying patients. Importantly, when excluding patients with UC and celiac disease, the specificity of anti-GP2 autoantibodies for CD is approximately 98% compared to that for non-intestinal diseases [100].
The use of a novel enzyme-linked immunosorbent assay (ELISA) utilizing recombinant GP2 as a solid-phase antigen has revealed the existence of both IgG and IgA anti-GP2 autoantibodies [96, 98]. The levels of these two antibodies have been associated with more complex intestinal behaviors, such as ileal involvement, strictures, penetrating behavior, and surgery in patients with CD. Patients with structuring behavior and fibrostenotic complications exhibit elevated levels of anti-GP2 IgG, whereas individuals with penetrating diseases show reduced levels of anti-GP2 IgG [101,102,103]. Positive anti-GP2 autoantibodies have been linked to distinct patient characteristics, including an earlier onset, lower incidence of isolated colonic disease, and higher incidence of stenotic behavior anti-GP2 autoantibodies negative ones [104]. Furthermore, in patients with coexisting celiac disease, elevated levels of anti-GP2 IgA were observed, which correlated significantly with celiac disease-specific antibodies such as anti-tissue transglutaminase (anti-tTG) and anti-endomysial IgA antibodies [99]. In a study involving 174 individuals with celiac disease and 84 patients on a gluten-free diet (GFD), significantly increased levels of anti-GP2 IgA were found and showed a strong association with levels of endomysial antibodies (EMA) and anti-tTG IgA antibodies [105]. Moreover, this study notably revealed, for the first time, a significant correlation between the levels of anti-GP2 IgA and the degree of mucosal damage [105]. Interestingly, the loss of tolerance to GP2 appears to be transient and disease-related, as it disappears under GFD, a trend that has also been corroborated in another article [105, 106]. Notably, CD patients with pouchitis exhibit elevated levels of anti-GP2 IgA [107], and elevated levels of anti-GP2 IgA autoantibodies have also been detected in a minority of UC patients, particularly those with primary sclerosing cholangitis (PSC) as an extraintestinal manifestation [108, 109]. Further studies comparing anti-GP2 antibody titers in serum samples from independent cohorts of patients with PSC showed that anti-GP2 IgA autoantibodies were linked to a lower survival rate and an increased risk of developing cholangiocarcinoma, a type of bile duct cancer [110]. Another report indicated that the simultaneous detection of both anti-GP2 isoform 1 IgG and isoform 4 IgA was an effective diagnostic marker for PSC complicated with cirrhosis, exhibiting a sensitivity of 66.0% and specificity of 97.9% (Youden index: 0.64) [109].
The autoimmune response to GP2 is predominantly associated with the ileal site of disease, with notably lower levels of anti-GP2 autoantibodies detected in CD patients with colon involvement than in those with ileal lesions [98, 101, 111]. Even in UC cases, which generally have a reduced incidence of anti-GP2 autoantibodies, these antibodies can be detected in the serum and feces of patients who develop pouchitis after colectomy [107]. Furthermore, studies have shown a significant association between anti-GP2 autoantibodies and younger age (<16 years old) in CD patients [101]. In conclusion, a growing body of evidence strongly supports the association between the level of anti-GP2 autoantibodies and the specific clinical phenotypes of CD.
The presence of different isoforms of GP2 has raised the possibility that autoantibodies induced by specific isoforms have distinct implications in the diagnosis and pathogenesis of IBD. Research has shown that certain isoforms of GP2 are linked to the generation of autoantibodies and their effects on the pathophysiology of IBD. Overexpression of pancreatic-, intestinal M cell-, and L cell-derived GP2 isoform 4 (but not isoform 2), induced by TNF-α, in the gut of CD patients with ileocolic manifestations was shown to lead to the production of anti-GP2 autoantibodies [12, 29]. These autoantibodies increase the adherence and invasion of FimH-positive pathobionts within the intestine, thereby promoting the pathophysiology of IBD [29]. It was found that the detection of autoantibodies against the four isoforms of GP2 had diagnostic value in distinguishing between pediatric patients with UC and CD [31]. Pediatric patients with UC and CD with at least 1 positive antibody against the 4 isoforms of GP2 had a sensitivity of 54% and a specificity of 84%. Among CD patients, 42% had more than one type of anti-GP2 subtype of autoantibody. Anti-GP2 isoform 1 IgG and isoform 4 IgA are diagnostically valuable for the differential diagnosis of CD. The sensitivity of the anti-GP2 isoform 4 IgG to CD was 38%. However, anti-GP2 isoform 4 IgG serves as a relatively stable marker independent of disease activity over time only in 5% of UC (95% specificity) [31]. In addition, anti-GP2 isoforms 3 and 4 IgG showed high accuracy in differentiating between UC and CD [31].
Notably, research has demonstrated the stability of these anti-GP2 antibodies over an extended period. It was shown that only 5% CD patients changed the anti-GP2 isoform 4 IgA and IgG status, as well as other anti-pancreatic autoantibodies [112]. These findings emphasize the potential diagnostic and prognostic value of analyzing anti-GP2 autoantibodies, particularly in relation to specific isoforms, in differentiating between UC and CD and assessing disease activity and progression over time.
Furthermore, studies have implicated the microbial composition and the presence of specific bacteria in the disruption of immune tolerance towards GP2 and the subsequent development of GP2-intolerant autoimmunity. In general, the human body maintains immune tolerance to GP2, and the immune system is educated to suppress autoimmune responses against GP2. This tolerance is achieved, in part, through the regulation of GP2 as an autoimmune regulator-dependent gene in thymic epithelial cells, as reported by Rattay et al. [113]. This suggests that the loss of GP2-related tolerance may be related to the initiation and triggering of intestinal inflammatory responses. In our search, we also observed a significant increase in luminal anti-GP2 IgG autoantibody, consistent with human patients, in DSS-treated WT mice. Coupled with the observed increase in serum anti-E. coli antibodies in DSS-treated GP2−/− mice, we determined that translocated GP2-binding bacteria may act as immune enhancers, promoting the development of an autoimmune response specifically targeting GP2 [12] (Fig. 1).
The role of GP2 in other diseases and its potential as a therapeutic target
In addition to anti-GP2 autoantibodies in IBD, the role of GP2-related gene mutations in diseases has also been reported in recent years. Data analysis based on information from gnomAD (v3.1.2) (https://gnomad.broadinstitute.org/) has revealed a total of 94 synonymous mutations, 194 missense/in-frame indel mutations, and 35 predicted loss-of-function (pLOF) mutations have been found in the GP2 gene. However, whether these mutations are pathogenic has rarely been studied. It was not until 2020 that a groundbreaking study based on a meta-analysis of three genome-wide association study (GWAS) datasets comprising 2039 pancreatic cancer patients and 32,592 controls from Japan confirmed the relatively pivotal role of GP2 gene variants in pancreatic cancer [63]. This study analyzed a total of 7,914,378 SNPs across the whole genome and revealed three genome-wide significant loci associated with pancreatic cancer risk in this population: 13q12.2, 13q22.1, and the previously unreported 16p12.3 locus. Of particular note, the lead SNP at 16p12.3, namely, rs78193826 (a missense variant, C>T: p.V432M), exhibited a 46% increased risk of pancreatic cancer associated with each minor T allele. Furthermore, rs78193826 appears to be Asian-specific (especially East Asian), as the frequency of risk allele is around 8% in the East-Asian population and around 3% in South-Asian population but only 0.1% in populations of European ancestry [114]. Functional studies conducted in GP2-expressing pancreatic cancer cell lines demonstrated that rs78193826 may impact the activity of KRAS, a key driver of pancreatic cancer (with mutation frequencies >93%) [115]. These findings are particularly noteworthy as they unveil an Asian-specific GP2 gene variant that was previously overlooked in Western populations. Additionally, considering that GP2 variants are associated with a range of diseases and conditions beyond pancreatic cancer, including body mass index (BMI) (rs12597579) [116], type 1 [117] and 2 diabetes (rs117267808) [118], acute myeloid leukemia [119], and sleep quality [120], this study revealed the pleiotropic effects of GP2 variants, suggesting their potential roles in metabolic traits such as type 2 diabetes [63, 118]. In fact, in a comprehensive GWAS involving Japanese subjects, the top 3 16p12.3 locus SNPs, including rs78193826, showed significant associations with type 2 diabetes, glycated hemoglobin (HbA1c), and blood glucose levels [121]. Moreover, Mendelian randomization (MR) analysis hinted at intriguing associations between HbA1c levels and pancreatic cancer risk, suggesting the presence of shared genetic susceptibility [63].
GP2, as a multifunctional protein, is believed to have potential therapeutic applications beyond its role as a diagnostic tool. For instance, as previously mentioned, GP2 serves as a specific marker for pancreatic acinar cells [23, 122]. Building upon this foundation, Ameri and colleagues successfully isolated and differentiated functional β cells capable of responding to changes in glucose levels from GP2+ cells [123]. These newly generated β cells exhibited the ability to produce insulin in a glucose-dependent manner, similar to healthy β cells, holding tremendous potential for diabetes treatment [123].
Furthermore, GP2 has been shown to effectively prevent the infiltration of E. coli into the intestinal mucosa [12]. Even during colitis when pancreatic GP2 secretion increases, there are still more bacteria that remain unbound to GP2, which may contribute to the worsening of the inflammation [12]. Therefore, recombinant GP2 protein has emerged as a potential therapeutic approach for addressing dysbiosis during the onset of IBD. Building upon this, we inoculated GFP-labeled E. coli treated with recombinant GP2 protein into GP2−/− mice [12]. The results demonstrated a significant reduction in the invasive capacity of the treated S17 in the mucosa, further validating the therapeutic potential of GP2.
Reducing the levels of anti-GP2 autoantibodies has also emerged as a potential therapeutic approach. Several studies have demonstrated the impact of microbes, particularly probiotics, on anti-GP2 autoantibody levels. It has been observed that untreated patients have higher levels of anti-GP2 autoantibodies than patients who have undergone treatment with probiotics, suggesting a potential role of microbial modulation in GP2-intolerant autoimmunity [107]. Further research is warranted to clarify the specific mechanisms underlying this process and explore potential therapeutic approaches targeting the modulation of the gut microbiome in the context of GP2-related autoimmune responses.
These exciting research findings provide a promising glimpse into the future of GP2’s therapeutic potential, successfully demonstrating the prospects of targeting GP2 in the treatment of autoimmune diseases and gastrointestinal disorders. However, some potential treatment modalities, such as recombinant GP2 protein formulations or interventions to inhibit autoantibody production, are still in the early stages of preclinical validation. Further research is needed to progress toward clinical validation, paving the way for more comprehensive studies and ultimately translating these innovative approaches into clinical practice.
Conclusion
In conclusion, GP2 is a widely distributed protein in the digestive tract that plays multiple roles in the mucosal barrier system and has been implicated in IBD. Its involvement in IBD pathogenesis and potential as a serological diagnostic marker highlight its significance in this disease. However, as a highly glycosylated protein with multiple subtypes, the synthesis, post-translational modifications, membrane localization, and release of GP2 are highly complex, leading to discrepancies among researchers regarding its physiological functions. Therefore, further comprehensive research on GP2 is critically needed to fully understand its potential and pave the way for the development of innovative diagnostic and therapeutic approaches targeting GP2 in treating IBD.
Data availability
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
Change history
22 April 2024
A Correction to this paper has been published: https://doi.org/10.1007/s00281-024-01002-z
References
Okumura R, Kurakawa T, Nakano T, Kayama H, Kinoshita M, Motooka D, Gotoh K, Kimura T, Kamiyama N, Kusu T, Ueda Y, Wu H, Iijima H, Barman S, Osawa H, Matsuno H, Nishimura J, Ohba Y, Nakamura S et al (2016) Lypd8 promotes the segregation of flagellated microbiota and colonic epithelia. Nature 532:117–121
Pott J, Hornef M (2012) Innate immune signalling at the intestinal epithelium in homeostasis and disease. EMBO Rep 13:684–698
Kurashima Y, Kiyono H (2017) Mucosal ecological network of epithelium and immune cells for gut homeostasis and tissue healing. Annu Rev Immunol 35:119–147
Kamioka M, Goto Y, Nakamura K, Yokoi Y, Sugimoto R, Ohira S, Kurashima Y, Umemoto S, Sato S, Kunisawa J, Takahashi Y, Domino SE, Renauld JC, Nakae S, Iwakura Y, Ernst PB, Ayabe T, Kiyono H (2022) Intestinal commensal microbiota and cytokines regulate Fut2(+) Paneth cells for gut defense. Proc Natl Acad Sci USA 119(3):e2115230119
Yeh TC, Wilson AC, Irwin DM (1993) Evolution of rodent lysozymes: isolation and sequence of the rat lysozyme genes. Mol Phylogenet Evol 2:65–75
Russell AB, LeRoux M, Hathazi K, Agnello DM, Ishikawa T, Wiggins PA, Wai SN, Mougous JD (2013) Diverse type VI secretion phospholipases are functionally plastic antibacterial effectors. Nature 496:508–512
Mukherjee S, Zheng H, Derebe MG, Callenberg KM, Partch CL, Rollins D, Propheter DC, Rizo J, Grabe M, Jiang Q-X, Hooper LV (2014) Antibacterial membrane attack by a pore-forming intestinal C-type lectin. Nature 505:103–107
Hand TW, Reboldi A (2021) Production and function of immunoglobulin A. Ann Rev Immunol 39:695–718
Zhang Z, Tanaka I, Pan Z, Ernst PB, Kiyono H, Kurashima Y (2022) Intestinal homeostasis and inflammation: gut microbiota at the crossroads of pancreas-intestinal barrier axis. Eur J Immunol 52:1035–1046
Jia W, Xie G, Jia W (2018) Bile acid-microbiota crosstalk in gastrointestinal inflammation and carcinogenesis. Nat Rev Gastroenterol Hepatol 15:111–128
Konturek SJ, Zabielski R, Konturek JW, Czarnecki J (2003) Neuroendocrinology of the pancreas; role of brain-gut axis in pancreatic secretion. Eur J Pharmacol 481:1–14
Kurashima Y, Kigoshi T, Murasaki S, Arai F, Shimada K, Seki N, Kim YG, Hase K, Ohno H, Kawano K, Ashida H, Suzuki T, Morimoto M, Saito Y, Sasou A, Goda Y, Yuki Y, Inagaki Y, Iijima H et al (2021) Pancreatic glycoprotein 2 is a first line of defense for mucosal protection in intestinal inflammation. Nat Commun 12:1067
Teratani T, Mikami Y, Nakamoto N, Suzuki T, Harada Y, Okabayashi K, Hagihara Y, Taniki N, Kohno K, Shibata S, Miyamoto K, Ishigame H, Chu PS, Sujino T, Suda W, Hattori M, Matsui M, Okada T, Okano H et al (2020) The liver-brain-gut neural arc maintains the T(reg) cell niche in the gut. Nature 585:591–596
Cai J, Sun L, Gonzalez FJ (2022) Gut microbiota-derived bile acids in intestinal immunity, inflammation, and tumorigenesis. Cell Host & Microbe 30:289–300
Doyle CJ, Yancey K, Pitt HA, Wang M, Bemis K, Yip-Schneider MT, Sherman ST, Lillemoe KD, Goggins MD, Schmidt CM (2012) The proteome of normal pancreatic juice. Pancreas 41:186–194
Ishimoto Y, Yamada K, Yamamoto S, Ono T, Notoya M, Hanasaki K (2003) Group V and X secretory phospholipase A(2)s-induced modification of high-density lipoprotein linked to the reduction of its antiatherogenic functions. Biochim Biophys Acta 1642:129–138
Shin JH, Seeley RJ (2019) Reg3 proteins as gut hormones? Endocrinology 160:1506–1514
Chairatana P, Chu H, Castillo PA, Shen B, Bevins CL, Nolan EM (2016) Proteolysis triggers self-assembly and unmasks innate immune function of a human alpha-defensin peptide. Chem Sci 7:1738–1752
Nishiyama H, Nagai T, Kudo M, Okazaki Y, Azuma Y, Watanabe T, Goto S, Ogata H, Sakurai T (2018) Supplementation of pancreatic digestive enzymes alters the composition of intestinal microbiota in mice. Biochem Biophys Res Commun 495:273–279
Mukherjee S, Partch CL, Lehotzky RE, Whitham CV, Chu H, Bevins CL, Gardner KH, Hooper LV (2009) Regulation of C-type lectin antimicrobial activity by a flexible N-terminal prosegment. J Biol Chem 284:4881–4888
MacDonald RJ, Ronzio RA (1972) Comparative analysis of zymogen granule membrane polypeptides. Biochem Biophys Res Commun 49:377–382
Hoops TC, Ivanov I, Cui Z, Colomer-Gould V, Rindler MJ (1993) Incorporation of the pancreatic membrane protein GP-2 into secretory granules in exocrine but not endocrine cells. J Biol Chem 268:25694–25705
Merz S, Breunig M, Melzer MK, Heller S, Wiedenmann S, Seufferlein T, Meier M, Kruger J, Mulaw MA, Hohwieler M, Kleger A (2023) Single-cell profiling of GP2-enriched pancreatic progenitors to simultaneously create acinar, ductal, and endocrine organoids. Theranostics 13:1949–1973
Ronzio RA, Kronquist KE, Lewis DS, MacDonald RJ, Mohrlok SH, O’Donnell JJ Jr (1978) Glycoprotein synthesis in the adult rat pancreas. IV. Subcellular distribution of membrane glycoproteins. Biochim Biophys Acta 508:65–84
Fukuoka S (1994) Analysis of ZAPs, zymogen granule membrane associated proteins, in the regulated exocytosis of the pancreas. Biosci Biotechnol Biochem 58:1282–1285
Terahara K, Yoshida M, Igarashi O, Nochi T, Pontes GS, Hase K, Ohno H, Kurokawa S, Mejima M, Takayama N, Yuki Y, Lowe AW, Kiyono H (2008) Comprehensive gene expression profiling of Peyer’s patch M cells, villous M-like cells, and intestinal epithelial cells. J Immunol 180:7840–7846
Hase K, Kawano K, Nochi T, Pontes GS, Fukuda S, Ebisawa M, Kadokura K, Tobe T, Fujimura Y, Kawano S, Yabashi A, Waguri S, Nakato G, Kimura S, Murakami T, Iimura M, Hamura K, Fukuoka S, Lowe AW et al (2009) Uptake through glycoprotein 2 of FimH(+) bacteria by M cells initiates mucosal immune response. Nature 462:226–230
Roggenbuck D, Goihl A, Sowa M, Lopens S, Rodiger S, Schierack P, Conrad K, Sommer U, Johrens K, Grutzmann R, Reinhold D, Laass MW (2023) Human glycoprotein-2 expressed in Brunner glands — a putative autoimmune target and link between Crohn’s and coeliac disease. Clin Immunol 247:109214
Derer S, Brethack AK, Pietsch C, Jendrek ST, Nitzsche T, Bokemeyer A, Hov JR, Schaffler H, Bettenworth D, Grassl GA, Sina C (2020) Inflammatory bowel disease-associated GP2 autoantibodies inhibit mucosal immune response to adherent-invasive bacteria. Inflamm Bowel Dis 26:1856–1868
Fukuoka S (2000) Molecular cloning and sequences of cDNAs encoding alpha (large) and beta (small) isoforms of human pancreatic zymogen granule membrane-associated protein GP2. Biochim Biophys Acta 1491:376–380
Rober N, Noss L, Goihl A, Reinhold D, Jahn J, de Laffolie J, Johannes W, Flemming GM, Roggenbuck D, Conrad K, Laass MW (2017) Autoantibodies against glycoprotein 2 isoforms in pediatric patients with inflammatory bowel disease. Inflamm Bowel Dis 23:1624–1636
Bork P, Sander C (1992) A large domain common to sperm receptors (Zp2 and Zp3) and TGF-beta type III receptor. FEBS Lett 300:237–240
Li KJ, Siao SC, Wu CH, Shen CY, Wu TH, Tsai CY, Hsieh SC, Yu CL (2014) EGF receptor-dependent mechanism may be involved in the Tamm-Horsfall glycoprotein-enhanced PMN phagocytosis via activating Rho family and MAPK signaling pathway. Molecules 19:1328–1343
Kolenda R, Burdukiewicz M, Schiebel J, Rodiger S, Sauer L, Szabo I, Orlowska A, Weinreich J, Nitschke J, Bohm A, Gerber U, Roggenbuck D, Schierack P (2018) Adhesion of Salmonella to pancreatic secretory granule membrane major glycoprotein GP2 of human and porcine origin depends on FimH sequence variation. Front Microbiol 9:1905
Lewis DS, MacDonald RJ, Kronquist KE, Ronzio RA (1977) Purification and partial characterization of an integral membrane glycoprotein from zymogen granules of dog pancreas. FEBS Lett 76:115–120
Havinga JR, Strous GJ, Poort C (1983) Biosynthesis of the major glycoprotein associated with zymogen-granule membranes in the pancreas. Eur J Biochem 133:449–454
Paquette J, Leblond FA, Beattie M, LeBel D (1986) Reducing conditions induce a total degradation of the major zymogen granule membrane protein in both its membranous and its soluble form. Immunochemical quantitation of the two forms. Biochem Cell Biol 64:456–462
Reggio HA, Palade GE (1978) Sulfated compounds in the zymogen granules of the guinea pig pancreas. J Cell Biol 77:288–314
Tartakoff AM, Jamieson JD, Scheele GA, Palade GE (1975) Studies on the pancreas of the guinea pig. Parallel processing and discharge of exocrine proteins. J Biol Chem 250:2671–2677
Scheele GA, Fukuoka S, Freedman SD (1994) Role of the GP2/THP family of GPI-anchored proteins in membrane trafficking during regulated exocrine secretion. Pancreas 9:139–149
Fritz BA, Lowe AW (1996) Polarized GP2 secretion in MDCK cells via GPI targeting and apical membrane-restricted proteolysis. Am J Physiol 270:G176–G183
Colomer V, Lal K, Hoops TC, Rindler MJ (1994) Exocrine granule specific packaging signals are present in the polypeptide moiety of the pancreatic granule membrane protein GP2 and in amylase: implications for protein targeting to secretory granules. EMBO J 13:3711–3719
Fritz BA, Poppel CS, Fei MW, Lowe AW (2002) Processing of the major pancreatic zymogen granule membrane protein, GP2. Pancreas 24:336–343
Maxwell SE, Ramalingam S, Gerber LD, Udenfriend S (1995) Cleavage without anchor addition accompanies the processing of a nascent protein to its glycosylphosphatidylinositol-anchored form. Proc Natl Acad Sci USA 92:1550–1554
Scheffer RC, Poort C, Slot JW (1980) Fate of the major zymogen granule membrane-associated glycoproteins from rat pancreas. A biochemical and immunocytochemical study. Eur J Cell Biol 23:122–128
Geuze HJ, Slot JW, van der Ley PA, Scheffer RC (1981) Use of colloidal gold particles in double-labeling immunoelectron microscopy of ultrathin frozen tissue sections. J Cell Biol 89:653–665
Lisanti MP, Sargiacomo M, Graeve L, Saltiel AR, Rodriguez-Boulan E (1988) Polarized apical distribution of glycosyl-phosphatidylinositol-anchored proteins in a renal epithelial cell line. Proc Natl Acad Sci USA 85:9557–9561
Brown DA, Crise B, Rose JK (1989) Mechanism of membrane anchoring affects polarized expression of two proteins in MDCK cells. Science 245:1499–1501
Freedman SD, Scheele GA (1993) Reversible pH-induced homophilic binding of GP2, a glycosyl-phosphatidylinositol-anchored protein in pancreatic zymogen granule membranes. Eur J Cell Biol 61:229–238
Freedman SD, Scheele GA (1993) Regulated secretory proteins in the exocrine pancreas aggregate under conditions that mimic the trans-Golgi network. Biochem Biophys Res Commun 197:992–999
Fukuoka S, Freedman SD, Scheele GA (1991) A single gene encodes membrane-bound and free forms of GP-2, the major glycoprotein in pancreatic secretory (zymogen) granule membranes. Proc Natl Acad Sci USA 88:2898–2902
Rindler MJ, Hoops TC (1990) The pancreatic membrane protein GP-2 localizes specifically to secretory granules and is shed into the pancreatic juice as a protein aggregate. Eur J Cell Biol 53:154–163
Fukuoka S, Freedman SD, Yu H, Sukhatme VP, Scheele GA (1992) GP-2/THP gene family encodes self-binding glycosylphosphatidylinositol-anchored proteins in apical secretory compartments of pancreas and kidney. Proc Natl Acad Sci USA 89:1189–1193
Freedman SD, Kern HF, Scheele GA (1998) Acinar lumen pH regulates endocytosis, but not exocytosis, at the apical plasma membrane of pancreatic acinar cells. Eur J Cell Biol 75:153–162
Freedman SD, Kern HF, Scheele GA (1998) Cleavage of GPI-anchored proteins from the plasma membrane activates apical endocytosis in pancreatic acinar cells. Eur J Cell Biol 75:163–173
Schmidt K, Dartsch H, Linder D, Kern HF, Kleene R (2000) A submembranous matrix of proteoglycans on zymogen granule membranes is involved in granule formation in rat pancreatic acinar cells. J Cell Sci 113(Pt 12):2233–2242
Kalus I, Hodel A, Koch A, Kleene R, Edwardson JM, Schrader M (2002) Interaction of syncollin with GP-2, the major membrane protein of pancreatic zymogen granules, and association with lipid microdomains. Biochem J 362:433–442
Jacob M, Laine J, LeBel D (1992) Specific interactions of pancreatic amylase at acidic pH. Amylase and the major protein of the zymogen granule membrane (GP-2) bind to immobilized or polymerized amylase. Biochem Cell Biol 70:1105–1114
Parker EM, Zaman MM, Freedman SD (2000) GP2, a GPI-anchored protein in the apical plasma membrane of the pancreatic acinar cell, co-immunoprecipitates with src kinases and caveolin. Pancreas 21:219–225
Yu S, Michie SA, Lowe AW (2004) Absence of the major zymogen granule membrane protein, GP2, does not affect pancreatic morphology or secretion. J Biol Chem 279:50274–50279
Dittie A, Kern HF (1992) The major zymogen granule membrane protein GP-2 in the rat pancreas is not involved in granule formation. Eur J Cell Biol 58:243–258
Hansen LJ, Reddy MK, Reddy JK (1983) Comparison of secretory protein and membrane composition of secretory granules isolated from normal and neoplastic pancreatic acinar cells of rats. Proc Natl Acad Sci USA 80:4379–4383
Lin Y, Nakatochi M, Hosono Y, Ito H, Kamatani Y, Inoko A, Sakamoto H, Kinoshita F, Kobayashi Y, Ishii H, Ozaka M, Sasaki T, Matsuyama M, Sasahira N, Morimoto M, Kobayashi S, Fukushima T, Ueno M, Ohkawa S et al (2020) Genome-wide association meta-analysis identifies GP2 gene risk variants for pancreatic cancer. Nat Commun 11:3175
Hoops TC, Rindler MJ (1991) Isolation of the cDNA encoding glycoprotein-2 (GP-2), the major zymogen granule membrane protein. Homology to uromodulin/Tamm-Horsfall protein. J Biol Chem 266:4257–4263
Rindler MJ, Naik SS, Li N, Hoops TC, Peraldi MN (1990) Uromodulin (Tamm-Horsfall glycoprotein/uromucoid) is a phosphatidylinositol-linked membrane protein. J Biol Chem 265:20784–20789
Saemann MD, Weichhart T, Zeyda M, Staffler G, Schunn M, Stuhlmeier KM, Sobanov Y, Stulnig TM, Akira S, von Gabain A, von Ahsen U, Horl WH, Zlabinger GJ (2005) Tamm-Horsfall glycoprotein links innate immune cell activation with adaptive immunity via a Toll-like receptor-4-dependent mechanism. J Clin Invest 115:468–475
Wu TH, Hsieh SC, Yu CY, Lee YF, Tsai CY, Yu CL (2008) Intact protein core structure is essential for protein-binding, mononuclear cell proliferating, and neutrophil phagocytosis-enhancing activities of normal human urinary Tamm-Horsfall glycoprotein. Int Immunopharmacol 8:90–99
Kobayashi K, Yanagihara K, Ishiguro K, Fukuoka S (2004) GP2/THP gene family of self-binding, GPI-anchored proteins forms a cluster at chromosome 7F1 region in mouse genome. Biochem Biophys Res Commun 322:659–664
Dou W, Thompson-Jaeger S, Laulederkind SJ, Becker JW, Montgomery J, Ruiz-Bustos E, Hasty DL, Ballou LR, Eastman PS, Srichai B, Breyer MD, Raghow R (2005) Defective expression of Tamm-Horsfall protein/uromodulin in COX-2-deficient mice increases their susceptibility to urinary tract infections. Am J Physiol Renal Physiol 289:F49–F60
Stsiapanava A, Xu C, Nishio S, Han L, Yamakawa N, Carroni M, Tunyasuvunakool K, Jumper J, de Sanctis D, Wu B, Jovine L (2022) Structure of the decoy module of human glycoprotein 2 and uromodulin and its interaction with bacterial adhesin FimH. Nat Struct Mol Biol 29:190–193
Pak J, Pu Y, Zhang ZT, Hasty DL, Wu XR (2001) Tamm-Horsfall protein binds to type 1 fimbriated Escherichia coli and prevents E. coli from binding to uroplakin Ia and Ib receptors. J Biol Chem 276:9924–9930
Bates JM, Raffi HM, Prasadan K, Mascarenhas R, Laszik Z, Maeda N, Hultgren SJ, Kumar S (2004) Tamm-Horsfall protein knockout mice are more prone to urinary tract infection: rapid communication. Kidney Int 65:791–797
Roggenbuck D, Hausdorf G, Martinez-Gamboa L, Reinhold D, Buttner T, Jungblut PR, Porstmann T, Laass MW, Henker J, Buning C, Feist E, Conrad K (2009) Identification of GP2, the major zymogen granule membrane glycoprotein, as the autoantigen of pancreatic antibodies in Crohn’s disease. Gut 58:1620–1628
Roggenbuck D, Reinhold D, Werner L, Schierack P, Bogdanos DP, Conrad K (2013) Glycoprotein 2 antibodies in Crohn’s disease. Adv Clin Chem 60:187–208
Juste C, Kreil DP, Beauvallet C, Guillot A, Vaca S, Carapito C, Mondot S, Sykacek P, Sokol H, Blon F, Lepercq P, Levenez F, Valot B, Carre W, Loux V, Pons N, David O, Schaeffer B, Lepage P et al (2014) Bacterial protein signals are associated with Crohn’s disease. Gut 63:1566–1577
Jostins L, Ripke S, Weersma RK, Duerr RH, McGovern DP et al (2012) Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 491:119–124
Darfeuille-Michaud A, Boudeau J, Bulois P, Neut C, Glasser AL, Barnich N, Bringer MA, Swidsinski A, Beaugerie L, Colombel JF (2004) High prevalence of adherent-invasive Escherichia coli associated with ileal mucosa in Crohn’s disease. Gastroenterology 127:412–421
Chassaing B, Rolhion N, de Vallee A, Salim SY, Prorok-Hamon M, Neut C, Campbell BJ, Soderholm JD, Hugot JP, Colombel JF, Darfeuille-Michaud A (2011) Crohn disease-associated adherent-invasive E. coli bacteria target mouse and human Peyer’s patches via long polar fimbriae. J Clin Invest 121:966–975
Yu S, Lowe AW (2009) The pancreatic zymogen granule membrane protein, GP2, binds Escherichia coli type 1 fimbriae. BMC Gastroenterol 9:58
Zeiner SA, Dwyer BE, Clegg S (2012) FimA, FimF, and FimH are necessary for assembly of type 1 fimbriae on Salmonella enterica serovar Typhimurium. Infect Immun 80:3289–3296
Werner L, Paclik D, Fritz C, Reinhold D, Roggenbuck D, Sturm A (2012) Identification of pancreatic glycoprotein 2 as an endogenous immunomodulator of innate and adaptive immune responses. J Immunol 189:2774–2783
Yamanaka H, Nakanishi T, Takagi T, Ohsawa M, Kubo N, Yamamoto N, Takemoto T, Ohsawa K (2015) Helicobacter sp. MIT 01-6451 infection during fetal and neonatal life in laboratory mice. Exp Anim 64:375–382
Yang JY, Lee YS, Kim Y, Lee SH, Ryu S, Fukuda S, Hase K, Yang CS, Lim HS, Kim MS, Kim HM, Ahn SH, Kwon BE, Ko HJ, Kweon MN (2017) Gut commensal Bacteroides acidifaciens prevents obesity and improves insulin sensitivity in mice. Mucosal Immunol 10:104–116
de Souza HL, de Carvalho VR, Romeiro FG, Sassaki LY, Keller R, Rodrigues J (2012) Mucosa-associated but not luminal Escherichia coli is augmented in Crohn’s disease and ulcerative colitis. Gut Pathog 4:21
Bartlitz C, Kolenda R, Chilimoniuk J, Grzymajlo K, Rodiger S, Bauerfeind R, Ali A, Tchesnokova V, Roggenbuck D, Schierack P (2022) Adhesion of enteropathogenic, enterotoxigenic, and commensal Escherichia coli to the major zymogen granule membrane glycoprotein 2. Appl Environ Microbiol 88:e0227921
Matsumura T, Sugawara Y, Yutani M, Amatsu S, Yagita H, Kohda T, Fukuoka S, Nakamura Y, Fukuda S, Hase K, Ohno H, Fujinaga Y (2015) Botulinum toxin A complex exploits intestinal M cells to enter the host and exert neurotoxicity. Nat Commun 6:6255
Holzl MA, Hofer J, Kovarik JJ, Roggenbuck D, Reinhold D, Goihl A, Gartner M, Steinberger P, Zlabinger GJ (2011) The zymogen granule protein 2 (GP2) binds to scavenger receptor expressed on endothelial cells I (SREC-I). Cell Immunol 267:88–93
Baumgart DC, Carding SR (2007) Inflammatory bowel disease: cause and immunobiology. Lancet 369:1627–1640
Broberger O, Perlmann P (1959) Autoantibodies in human ulcerative colitis. J Exp Med 110:657–674
Walker JE (1978) Possible diagnostic test for Crohn’s disease by use of buccal mucosa. Lancet 2:759–760
Martin-de-Carpi J, Moriczi M, Pujol-Muncunill G, Navas-Lopez VM (2017) Pancreatic involvement in pediatric inflammatory bowel disease. Front Pediatr 5:218
Goischke EM, Zilly W (1992) Clinical importance of organ-specific antibodies in ulcerative colitis and Crohn disease. Z Gastroenterol 30:319–324
Spiess SE, Braun M, Vogelzang RL, Craig RM (1992) Crohn’s disease of the duodenum complicated by pancreatitis and common bile duct obstruction. Am J Gastroenterol 87:1033–1036
Desplat-Jego S, Johanet C, Escande A, Goetz J, Fabien N, Olsson N, Ballot E, Sarles J, Baudon JJ, Grimaud JC, Veyrac M, Chamouard P, Humbel RL (2007) Update on anti-Saccharomyces cerevisiae antibodies, anti-nuclear associated anti-neutrophil antibodies and antibodies to exocrine pancreas detected by indirect immunofluorescence as biomarkers in chronic inflammatory bowel diseases: results of a multicenter study. World J Gastroenterol 13:2312–2318
Seibold F, Mork H, Tanza S, Muller A, Holzhuter C, Weber P, Scheurlen M (1997) Pancreatic autoantibodies in Crohn’s disease: a family study. Gut 40:481–484
Seibold F, Weber P, Jenss H, Wiedmann KH (1991) Antibodies to a trypsin sensitive pancreatic antigen in chronic inflammatory bowel disease: specific markers for a subgroup of patients with Crohn’s disease. Gut 32:1192–1197
Bogdanos DP, Rigopoulou EI, Smyk DS, Roggenbuck D, Reinhold D, Forbes A, Laass MW, Conrad K (2011) Diagnostic value, clinical utility and pathogenic significance of reactivity to the molecular targets of Crohn’s disease specific-pancreatic autoantibodies. Autoimmun Rev 11:143–148
Pavlidis P, Forbes A, Bogdanos DP (2011) Antibodies to glycoprotein 2 (GP2) in patients with inflammatory bowel diseases from UK. Clin Chim Acta 412:1163–1164
Bonaci-Nikolic B, Spuran M, Andrejevic S, Nikolic M (2012) Autoantibodies to GP2, the major zymogen granule membrane glycoprotein, in patients with gluten-sensitive enteropathy: a possible serological trap. Clin Chim Acta 413:822–823
Fasano A (2012) Leaky gut and autoimmune diseases. Clin Rev Allergy Immunol 42:71–78
Bogdanos DP, Roggenbuck D, Reinhold D, Wex T, Pavlidis P, von Arnim U, Malfertheiner P, Forbes A, Conrad K, Laass MW (2012) Pancreatic-specific autoantibodies to glycoprotein 2 mirror disease location and behaviour in younger patients with Crohn’s disease. BMC Gastroenterol 12:102
Wells AD, McMillan I, Price AB, Ritchie JK, Nicholls RJ (1991) Natural history of indeterminate colitis. Br J Surg 78:179–181
Conrad K, Schmechta H, Klafki A, Lobeck G, Uhlig HH, Gerdi S, Henker J (2002) Serological differentiation of inflammatory bowel diseases. Eur J Gastroenterol Hepatol 14:129–135
Michaels MA, Jendrek ST, Korf T, Nitzsche T, Teegen B, Komorowski L, Derer S, Schroder T, Baer F, Lehnert H, Buning J, Fellerman K, Sina C (2015) Pancreatic autoantibodies against CUZD1 and GP2 are associated with distinct clinical phenotypes of Crohn’s disease. Inflamm Bowel Dis 21:2864–2872
Laass MW, Rober N, Range U, Noss L, Roggenbuck D, Conrad K (2015) Loss and gain of tolerance to pancreatic glycoprotein 2 in celiac disease. PLoS One 10:e0128104
Gross S, Bakker SF, van Bodegraven AA, van Hoogstraten IM, Gelderman KA, Bouma G, Mulder CJ, von Blomberg BM, Bontkes HJ (2014) Increased IgA glycoprotein-2 specific antibody titres in refractory celiac disease. J Gastrointestin Liver Dis 23:127–133
Werner L, Sturm A, Roggenbuck D, Yahav L, Zion T, Meirowithz E, Ofer A, Guzner-Gur H, Tulchinsky H, Dotan I (2013) Antibodies against glycoprotein 2 are novel markers of intestinal inflammation in patients with an ileal pouch. J Crohns Colitis 7:e522–e532
Kovacs G, Sipeki N, Suga B, Tornai T, Fechner K, Norman GL, Shums Z, Antal-Szalmas P, Papp M (2018) Significance of serological markers in the disease course of ulcerative colitis in a prospective clinical cohort of patients. PLoS One 13:e0194166
Sowa M, Kolenda R, Baumgart DC, Pratschke J, Papp M, Tornai T, Suchanski J, Bogdanos DP, Mytilinaiou MG, Hammermann J, Laass MW, Conrad K, Schramm C, Franke A, Roggenbuck D, Schierack P (2018) Mucosal autoimmunity to cell-bound GP2 isoforms is a sensitive marker in PSC and associated with the clinical phenotype. Front Immunol 9:1959
Jendrek ST, Gotthardt D, Nitzsche T, Widmann L, Korf T, Michaels MA, Weiss KH, Liaskou E, Vesterhus M, Karlsen TH, Mindorf S, Schemmer P, Bar F, Teegen B, Schroder T, Ehlers M, Hammers CM, Komorowski L, Lehnert H et al (2017) Anti-GP2 IgA autoantibodies are associated with poor survival and cholangiocarcinoma in primary sclerosing cholangitis. Gut 66:137–144
Somma V, Ababneh H, Ababneh A, Gatti S, Romagnoli V, Bendia E, Conrad K, Bogdanos DP, Roggenbuck D, Ciarrocchi G (2013) The novel Crohn’s disease marker anti-GP2 antibody is associated with ileocolonic location of disease. Gastroenterol Res Pract 2013:683824
Papp M, Sipeki N, Tornai T, Altorjay I, Norman GL, Shums Z, Roggenbuck D, Fechner K, Stocker W, Antal-Szalmas P, Veres G, Lakatos PL (2015) Rediscovery of the anti-pancreatic antibodies and evaluation of their prognostic value in a prospective clinical cohort of Crohn's patients: the importance of specific target antigens [GP2 and CUZD1]. J Crohns Colitis 9:659–668
Rattay K, Meyer HV, Herrmann C, Brors B, Kyewski B (2016) Evolutionary conserved gene co-expression drives generation of self-antigen diversity in medullary thymic epithelial cells. J Autoimmun 67:65–75
Lin Y, Nakatochi M, Sasahira N, Ueno M, Egawa N, Adachi Y, Kikuchi S (2021) Glycoprotein 2 in health and disease: lifting the veil. Genes Environ 43:53
Cancer Genome Atlas Research Network. Electronic address aadhe, Cancer Genome Atlas Research N (2017) Integrated genomic characterization of pancreatic ductal adenocarcinoma. Cancer Cell 32(185-203):e13
Wen W, Cho YS, Zheng W, Dorajoo R, Kato N, Qi L, Chen CH, Delahanty RJ, Okada Y, Tabara Y, Gu D, Zhu D, Haiman CA, Mo Z, Gao YT, Saw SM, Go MJ, Takeuchi F, Chang LC et al (2012) Meta-analysis identifies common variants associated with body mass index in east Asians. Nat Genet 44:307–311
Barrett JC, Clayton DG, Concannon P, Akolkar B, Cooper JD, Erlich HA, Julier C, Morahan G, Nerup J, Nierras C, Plagnol V, Pociot F, Schuilenburg H, Smyth DJ, Stevens H, Todd JA, Walker NM, Rich SS, Type 1 Diabetes Genetics C (2009) Genome-wide association study and meta-analysis find that over 40 loci affect risk of type 1 diabetes. Nat Genet 41:703–707
Suzuki K, Akiyama M, Ishigaki K, Kanai M, Hosoe J, Shojima N, Hozawa A, Kadota A, Kuriki K, Naito M, Tanno K, Ishigaki Y, Hirata M, Matsuda K, Iwata N, Ikeda M, Sawada N, Yamaji T, Iwasaki M et al (2019) Identification of 28 new susceptibility loci for type 2 diabetes in the Japanese population. Nat Genet 51:379–386
Choi H, Jung C, Sohn SK, Kim S, Kim HJ, Kim YK, Kim T, Zhang Z, Shin ES, Lee JE, Moon JH, Kim SH, Kim KH, Mun YC, Kim H, Park J, Kim J, Kim D (2013) Genome-wide genotype-based risk model for survival in acute myeloid leukaemia patients with normal karyotype. Br J Haematol 163:62–71
Jones SE, van Hees VT, Mazzotti DR, Marques-Vidal P, Sabia S, van der Spek A, Dashti HS, Engmann J, Kocevska D, Tyrrell J, Beaumont RN, Hillsdon M, Ruth KS, Tuke MA, Yaghootkar H, Sharp SA, Ji Y, Harrison JW, Freathy RM et al (2019) Genetic studies of accelerometer-based sleep measures yield new insights into human sleep behaviour. Nat Commun 10:1585
Kanai M, Akiyama M, Takahashi A, Matoba N, Momozawa Y, Ikeda M, Iwata N, Ikegawa S, Hirata M, Matsuda K, Kubo M, Okada Y, Kamatani Y (2018) Genetic analysis of quantitative traits in the Japanese population links cell types to complex human diseases. Nat Genet 50:390–400
Cogger KF, Sinha A, Sarangi F, McGaugh EC, Saunders D, Dorrell C, Mejia-Guerrero S, Aghazadeh Y, Rourke JL, Screaton RA, Grompe M, Streeter PR, Powers AC, Brissova M, Kislinger T, Nostro MC (2017) Glycoprotein 2 is a specific cell surface marker of human pancreatic progenitors. Nat Commun 8:331
Ameri J, Borup R, Prawiro C, Ramond C, Schachter KA, Scharfmann R, Semb H (2017) Efficient generation of glucose-responsive beta cells from isolated GP2(+) human pancreatic progenitors. Cell Rep 19:36–49
Acknowledgements
Figures were produced using Servier Medical Art (http://smart.servier.com/), licensed under a Creative Common Attribution 3.0 Generic License (https://creativecommons.org/licenses/by/3.0/).
Funding
This work was supported by Japan Society for the Promotion of Science (JSPS) Grant-in-Aid for Scientific Research S (18H05280 to H. K., 23H02699 to Y. K.); JSPS Fund for the Promotion of Joint International Research (18KK0432 to Y. K.); Japan Agency for Medical Research and Development (AMED) (JP223fa627003, JP21am0401029, JP223fa627003 to H. K.); (223fa627003h0001 to R. N.-O., Y. K., and H. K.); AMED Project Focused on Developing Key Technology for Discovering and Manufacturing Drugs for Next-Generation Treatment and Diagnosis (NeDDTrim) (JP21ae0121040 to H. K.), PRIME (20gm6010012h0004/20gm6210024h0001); and Japan Initiative for World-leading Vaccine Research and Development Centers (JP223fa627003 to H. K. and Y. K.); cSIMVA Vaccine Challenge Grants (to Z. Z.); Future Medicine Fund of Chiba University (to Y. K.); the Chiba University-UC San Diego Center for Mucosal Immunology, Allergy, and Vaccines (CMAV) (to Y. K., P. E., and H. K.); the Yamada Science Foundation (to Y. K.); and a 3M donation (to H. K.), JST SPRING, Grant Number JPMJSP2109 (to Z. Z.).
Author information
Authors and Affiliations
Contributions
Z. Z., Y. K., and I. T. conceived the idea for the review. Z. Z. and Y. K. wrote the manuscript with constructive input from R. N., P. B. E., and H. K. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
H. K. is the director and founder of HanaVax Inc. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
This article is a contribution to the Article Collection on Immunopathology of Barrier Function - Guest Editor: Koji Hase & Hiroshi Ohno
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: Funding information was incomplete and has been corrected.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, Z., Tanaka, I., Nakahashi-Ouchida, R. et al. Glycoprotein 2 as a gut gate keeper for mucosal equilibrium between inflammation and immunity. Semin Immunopathol (2024). https://doi.org/10.1007/s00281-023-00999-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00281-023-00999-z