Abstract
Mycobacterium tuberculosis remains the leading cause of death attributed to a single infectious organism. Bacillus Calmette-Guerin (BCG), the standard vaccine against M. tuberculosis, is thought to prevent only 5% of all vaccine-preventable deaths due to tuberculosis, thus an alternative vaccine is required. One of the principal barriers to vaccine development against M. tuberculosis is the complexity of the immune response to infection, with uncertainty as to what constitutes an immunological correlate of protection. In this paper, we seek to give an overview of the immunology of M. tuberculosis infection, and by doing so, investigate possible targets of vaccine development. This encompasses the innate, adaptive, mucosal and humoral immune systems. Though MVA85A did not improve protection compared with BCG alone in a large-scale clinical trial, the correlates of protection this has revealed, in addition to promising results from candidate such as VPM1002, M72/ASO1E and H56:IC31 point to a brighter future in the field of TB vaccine development.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Consigned to history in the minds of many, tuberculosis (TB) is a disease far from defeated. The pathogen responsible, Mycobacterium tuberculosis, is the leading cause of death attributed to a single infectious organism [1]. Administered at birth as part of the Expanded Programme on Immunization (EPI) since 1974 [2], the vaccine Bacillus Calmette-Guerin (BCG) currently has 90% coverage globally. Despite this, one person dies of TB every 20 s [3]. The efficacy of BCG varies from 0 to 80% in protecting against pulmonary TB [4]. It is estimated that globally, BCG prevents only 5% of all vaccine-preventable deaths due to TB [5], the cruel irony being that BCG is least effective in the areas of the world where it is most needed. It is also these very areas where HIV infection rates are highest, a cohort for whom BCG is contraindicated, and for whom the risks of TB are higher. In areas where efficacy is preserved however, protection can be durable, with protective efficacy of over 50 years demonstrated in an Alaskan population [6].
Following the disappointing results from the first phase 2b efficacy trial with a new-generation subunit vaccine, MVA85A, the TB vaccine field had a period of review and reflection [7]. Even with the more promising results recently demonstrated with another subunit vaccine, M72/AS01E [8], it is clear that the understanding of immune correlates of protection was, and still is, insufficient. In this review, we have focussed on the current understanding of TB immunology, and how this knowledge can be utilised in the development of novel vaccines.
BCG and efficacy
The derivation of BCG is a result of pathogenesis experiments carried out by Calmette and Guerin [9], who after 230 passages and 13 years declared the organism to be safe and protective against M. tuberculosis [10]. Major trials were initiated in the 1950s in the UK [11] and USA [12, 13], the results of which would set the tone for BCG trials of the future, in that the results were conflicting. The UK study involved 54,239 school children aged 14–15 years who were followed up for 20 years and demonstrated a protective efficacy of 77% [11]. In contrast, US trials involving 191,827 Puerto Rican school children aged 1–18 years [13] and 64,136 children aged above 5 years in Georgia and Alabama [12] showed a protective efficacy of only 16 and 29%, respectively, after 20 years follow-up. A critical moment in the acceptance of the variability in BCG efficacy came in 1980 with the publishing of the Chingleput trial [14]. Carried out in India and involving 73,459 individuals, in a highly surprising result, BCG provided no protection against TB [14]. Up to this point, much of the variance in reported efficacy had been put down to differences in trial design [15].
Though declared by Calmette to be a ‘virus fixe’ [16], multiple BCG strains have diverged [17]. Despite this, the impact of this divergence is insufficient to explain the variation in efficacy observed [18, 19]. The most significant factor influencing BCG efficacy that is supported by data is exposure to non-tuberculous mycobacteria (NTM) [19]. BCG efficacy correlates with latitude, as does NTM exposure, with BCG efficacy higher in temperate climates with lower NTM exposure [19]. NTM exposure is higher in tropical areas with lower BCG efficacy, such as Chingleput [20].
Although administering BCG vaccination shortly after birth reduces the risk of prior NTM exposure, such timing is frequently not possible in resource poor settings, particularly rural ones, and in areas where HIV infection needs to be excluded prior to BCG administration. A study from The Gambia has previously demonstrated NTM exposure in BCG-naive 4-month-old infants [21]. A study in mice has demonstrated that exposure to NTM post-BCG vaccination can reduce the protective efficacy of BCG [22].
There are two hypotheses proposed for the mechanism by which NTM exposure reduces BCG efficacy: masking and blocking [23, 24]. The masking hypothesis states that background immunity generated by prior exposure to NTM means that any added benefit of subsequent BCG vaccination is minimal [23]. The blocking hypothesis suggests that the pre-existing immune response to antigens shared by NTM and M. tuberculosis results in rapid eradication of BCG, such that the amount of available BCG derived antigens is limited, diminishing immunity [24]. Whilst these two hypotheses are not mutually exclusive, mathematical analysis of the BCG-REVAC trial suggests that blocking is the main mechanism [25].
One implication of this for the development of novel vaccines is that a dominant blocking mechanism suggests that a new vaccine need only be as good as BCG to have measurable effect, as long as it is not blocked by prior sensitisation [26]. Importantly, pre-exposure to NTM does not seem to affect the efficacy of non-replicating subunit vaccines [24].
The early innate response to M. tuberculosis exposure
The role of an early innate immune response in preventing or clearing early M. tuberculosis infection is increasingly recognised [27]. A subset of people exposed to M. tuberculosis are capable of achieving sterilising immunity post-exposure, termed early clearance [28]. The presence of latent M. tuberculosis infection (LTBI) is usually assessed via a tuberculin skin test (TST) or interferon-gamma release assay (IGRA), which when positive indicates immune sensitisation to M. tuberculosis, i.e. that an infection has occurred. In studies with a minimum of 2 years of longitudinal observation, the frequency of early clearance in household contacts of TB patients ranged from 3.4 to 26.8% when using TST conversion [28,29,30], whilst another study utilising IGRA conversion suggested 58% clearance [28]. A number of immune mechanisms for this have been proposed, including innate immune responses [31], antibody-innate cell interaction via Fc receptors [32] and lung resident T cells [33]. Investigations into a genetic basis for early clearance have found single nucleotide polymorphisms in NOD, and NRAMP1, suggesting a role for innate immunity in this process [34]. NOD2 signalling is already known to be increased post-BCG by up to a year through trained immunity [35].
The different components of an innate immune response to M. tuberculosis exposure are outlined below. Whilst vaccine development has traditionally focussed on the induction of an adaptive immune response, adjuvants that modulate innate immune pathways and a vaccine delivered by aerosol to the respiratory mucosa might target these pathways [36].
Airway epithelial cells
M. tuberculosis enters the body via small aerosolised droplets, inhaled into the airways [37, 38]. Though alveolar macrophages (AMs) are the principal target of these bacilli, they are also capable of infecting human lung epithelial cells [39]. Airway epithelial cells (AECs) express a variety of pattern recognition receptors (PRRs) in addition to surfactant proteins that bind components of the mycobacterial cell wall [40]. This epithelial recognition of M. tuberculosis activates a number of signalling pathways, inducing the production of cytokines such as tissue necrosis factor α (TNFα) and interferon-ɣ (IFN-ɣ), and chemokines such as IL-6 and IL-8 [41,42,43,44]. Furthermore, AECs are potent responders to cytokines such as IL-1β and type 1 interferons released by infected macrophages, enabling efficient cross-talk [31]. AECs are even capable of directly presenting intracellular antigens to resident CD8+ T cells via MHC class I molecules, stimulating IFN-ɣ production [45].
Alveolar macrophages
AMsare some of the first cells of the immune system to come into contact with M. tuberculosis, phagocytosing the bacilli. This phagocytosis is mediated principally by complement receptor 4 (CR4) [46]; however, AMs are highly heterogeneous in their phagocytic potential, with only 20% of AMs in culture becoming infected by M. tuberculosis even with high bacterial loads [47]. M. tuberculosis infection induces a phenotypic shift from oxidative phosphorylation (M2, anti-inflammatory) to aerobic glycolysis (M1, pro-inflammatory), resulting in increased IL-1β levels and decreased IL-10 levels [48]. This polarisation to an M1 phenotype aids antimicrobial activity [49]. However, the TNF produced by AMs may be counterproductive, with exogenous application of TNF increasing both intracellular bacterial load and the number of infected AMs [47, 49].
In a mouse model, the depletion of macrophages prior to a lethal infection with M. tuberculosis improved survival [50], yet specifically depleting activated macrophages was detrimental [51]. The protective effect of AMs may be dependent on their subtype.
Neutrophils
Another cell type implicated in the initial response to M. tuberculosis exposure are neutrophils, among the first immune cells to migrate to the site of infection [52]. Neutrophils secrete antimicrobial enzymes such as α-defensins and lactoferin [53], chemokines such as IP-10 [54] and MCP-1 [55] and cytokines such as TNFα [56]. Neutrophils kill M. tuberculosis primarily through the respiratory burst and phagocytosis [57]. Whilst this response may appear to be beneficial, the reality is more complex, as is exemplified in the case of lipocalin-2. Lipocalin-2 is a constituent of neutrophil secondary granules, blocking bacterial scavenging of iron [58]. In mice, lipocalin-2 increases susceptibility to M. tuberculosis prior to granuloma formation [59], potentially via increasing the amount of iron available to intracellular mycobacteria [59].
In humans, peripheral neutrophilia is a hallmark of TB disease and is a poor predictor of outcome [60], with neutrophil depletion decreasing M. tuberculosis killing [57]. A neutrophil-driven interferon inducible gene profile consisting of both IFN-ɣ and IFN-αβ was one of the principal components of an 86 transcript signature of active TB [61]. As a predominant cell type infected by M. tuberculosis, the evidence suggests a role for neutrophils in the pathogenesis of TB, a possible granulocytic Trojan horse [62].
Other innate cells
There are many other innate cell types for which there is some evidence for a role in protection against mycobacterial infection, including NK cells [63, 64], ɣδ T cells [65,66,67] and mucosal-associated invariant T cells [68, 69]. Innate lymphoid cells (ILCs) share features of both the innate and adaptive systems, and are categorised into three subsets [70]. Group 3 ILCs (ILC3s) mediate early protective immunity against M. tuberculosis, recruited via a CXCL13-CXCR5 axis to inducible bronchus-associated lymphoid tissue (iBALT)-associated granulomas [71].
iBALT, like other lymphoid organs, are composed of segregated T and B cell areas [72]. These highly organised structures form spontaneously in response to pulmonary infection [73]. iBALT surrounds the granulomas in M. tuberculosis infected humans [74], NHPs [75] and mice [76]. The absence of iBALT is associated with active disease, whereas presence is associated with containment of infection and maintenance of latency [74, 77].
ILC3 produce IL-17 and IL-22 in response to IL-23 stimulation [78], the IL-23 produced by M. tuberculosis infected lung cells [71]. Early neutralisation of IL-23 in mice increased early M. tuberculosis burden, resulting in a decreased formation of iBALT, whilst mice lacking ILC3 exhibit a reduction in the accumulation of early alveolar macrophages [71]. CXCL13 is induced in the lungs during M. tuberculosis infection, recruiting lymphocytes through CXCR5 to mediate their spatial organisation within iBALT [77]. IL-17 is one of the key mediators of increased CXCL13 levels [79] and will be covered later in the section on mucosal immunity. An overview of this axis can be seen in Fig. 1.
Using a mycobacterial growth inhibition assay, a new subset of innate cell has been found to be strongly associated with trained innate immunity [80]. Control of mycobacterial growth was associated with the presence of a non-classical CD14-dim monocyte population [80]. These cells are highly motile and able to release multiple cytokines, yet are weakly phagocytic [81]. One of the chemokines secreted includes CXCL10 (a CXCR3 ligand), production of which correlating strongly with BCG growth reduction [80]. Importantly, CXCR3 ligands such as CXCL10 are associated with trained immunity [80].
Trained immunity
Trained immunity describes epigenetic changes to the genes of the innate immune system, resulting in a memory like function. BCG vaccination has been shown to alter the acetylation and methylation of innate immune genes, amplifying the response to subsequent stimulus [82]. This may be the basis of the reported non-specific protective effect of BCG, which in some studies has been suggested to reduce mortality in the first 6–12 months of life [83]. This trained immunity induced by BCG can confer heterologous protection against other pathogens in vitro [35]. BCG induced a two-fold increase in monocyte derived cytokines such as IL-1β and TNFα in response to an in vitro bacterial and fungal challenge. Whilst for the most part this is a fairly short lived response, intravenous (IV) BCG has been shown to drive epigenetic changes in haematopoietic stem cells (HSCs), thus potentially impacting immunity over the longer term [84].
Harnessing the innate immune system
Targeting dendritic cells and haematopoietic stem cells
Activation of the adaptive immune response requires the presentation of M. tuberculosis antigens to T cells by dendritic cells and macrophages. There is a significant delay between infection and the induction of an adaptive immune response, which in humans is first detectable 18–20 days after infection [85]. Migration of M. tuberculosis to the draining lymph nodes only occurs 7–9 days post-infection; this delay allows for a marked expansion in bacterial population [85].
Methods of activating dendritic cells (DCs) via adjuvants may improve the protective efficacy of vaccines. Amphiphilic-CpG (amph-CpG), a modified TLR9 agonist in mice, enhances the T cell response to peptide vaccination in addition to upregulating CD103 [86]. When used in combination with Fgk4.5, an agonistic CD40 antibody, DCs are activated resulting in early M. tuberculosis control [87]. The use of TLR ligands requires care however, with the administration of polyl:C (a TLR3 ligand) exacerbating inflammation and increasing M. tuberculosis burden [88].
Intravenous BCG vaccination is capable of priming HSCs, resulting in a polarisation towards the myeloid lineage, correlating with an upregulation of IFN-ɣ [84]. This long lasting effect of IV BCG on the innate immune system may explain the superior protection seen in NHPs using this route of vaccination [89, 90]. Whilst this work provides important proof of concept for immune mechanistic work, such a route is unlikely to be a feasible approach for neonates or in low resource settings.
Targeting macrophages
M. tuberculosis has a variety of means to mitigate its destruction by macrophages, enabling it to survive in macrophages without destruction. An overview of this can be seen above in Fig. 2 [91,92,93,94,95,96,97,98,99,100,101,102]. The immune evasion and immunosuppression of macrophages by M. tuberculosis result in less effective antigen presentation, resulting in a less-effective adaptive immune response [103]. Increasing the rate of apoptosis would result in the release of apoptotic vesicles carrying M. tuberculosis to uninfected DCs, thus allowing antigen presentation and activating adaptive immunity [104].
M. tuberculosis inhibits inflammasome activation and IL-1β processing through zinc metalloproteinase 1 (zmp1) [91]. A recombinant BCG vaccine candidate in late preclinical development, BCG ∆zmp1, demonstrates increased phagosome maturation and phagolysosmal fusion, aiding antigen presentation resulting in improved protection [105,106,107].
Negating the capacity of M. tuberculosis to inhibit and neutralise reactive oxygen species (ROS) production by macrophages is yet another target for vaccine design. In doing so, macrophages would be better able to present antigens, thus improving immunogenicity and efficacy [108,109,110,111]. SigH is among these potential targets, involved in orchestrating an M. tuberculosis stress-response pathway [92]. A duplication of SigH in a majority of BCG strains further reduces the susceptibility of BCG to ROS [112]. The vaccine candidate M. tuberculosis∆sigH shows greater levels of apoptosis and a markedly stronger innate immune response [113], with sterile protection observed in some TB lesions in preclinical studies [113].
In addition to inhibiting phagolysosomal maturation/fusion and ROS inhibition, M. tuberculosis is also capable of inhibiting the maturation and trafficking of MHC molecules [114]. The activation of TLR2 by mycobacterial lipoproteins induces excessive signalling, resulting in an inhibition of MHC II expression [93,94,95]. A reduction in cathepsin S (Cat-S) expression is seen in M. tuberculosis infected macrophages [96], associated with the induction of IL-10 [97]. Addition of anti-IL-10 antibodies has been shown to restore macrophage Cat S expression, increasing antigen presentation [97]. BCG-CatS is a recombinant BCG vaccine engineered to secrete active Cat S, and stimulates much stronger macrophage presentation of Ag85B to CD4+ T cells than BCG [115].
Another strategy may be to target the autophagy and apoptosis pathways. M. tuberculosis inhibits autophagy through ESAT6, secreted by the ESX-1 secretion system [98]. As this is encoded in RD1 (region of difference 1), which is absent in BCG [116], autophagosome maturation is not inhibited with BCG [117]. A recombinant strain of BCG expressing ESX1 (BCG::ESX-1) is more protective than wild type BCG in mouse and guinea pig models, although this recombinant strain is more virulent [118]. By utilising the evolutionarily more distant ESX-1 from M.marinum, BCG::ESX-1Mmar has comparable efficacy and immunogenicity to BCG::ESX-1M. tuberculosis, yet has a safety profile comparable with BCG Pasteur in preclinical models [119].
VPM1002
The most clinically advanced recombinant BCG (rBCG) strain in development is rBCG ∆ureC::Hly, also known as VPM1002. This construct replaces the urease C gene with that of listeriolysin O, a haemolytic (hly) pore forming protein originating from Listeria monocytogenes [120, 121]. Listeriolysin O forms transmembrane β-barrel pores in the phagolysosome membrane, thus allowing the escape of antigens and mycobacterial DNA into the cytosol [122, 123]. By replacing urease C, the BCG construct is less able to alkalinise the phagolysosome, ensuring the activation of listeriolyin, which is active at a pH of 5.5 [124]. The net effect is designed to increase the levels of apoptosis, autophagy and inflammasome activation [125].
In mice, VPM1002 was cleared faster than BCG [126] and was also safer in immunodeficient SCID mice [121]. In both guinea pigs and non-human primates, the safety profile of VPM1002 has been found to be comparable with that seen with BCG [127, 128]. Grode et al. found VPM1002 to have greater protective efficacy compared with BCG in BALB/c mice [121]. Phases I and IIa studies found VPM1002 to be safe and capable of eliciting a strong immune response, at least comparable with BCG [129, 130].
A phase IIb trial in South Africa, evaluating the safety and immunogenicity of VPM1002 in comparison with BCG in both HIV unexposed and HIV-exposed uninfected (HEU) BCG naïve newborns (NCT02391415), has now concluded with data awaiting public release. A phase III trial is also underway in India (NCT03152903), investigating efficacy against relapse in adolescents and adults who have been recently treated for active TB.
The importance of a Th1 adaptive immune response
IFN-ɣ, the hallmark cytokine of a pro-inflammatory Th1 response, is critical for protection against M. tuberculosis [131, 132]. Individuals who are CD4+ T cell deficient, such as those infected with HIV, or those with inborn genetic errors of IFN-ɣ signalling are highly susceptible to M. tuberculosis, thus indicating the importance of the Th1 response [133,134,135]. Whilst essential to controlling M. tuberculosis infection, IFN-ɣ may not be sufficient [136]. Deficiency in other factors such as IL-1, IL-6 and TNFα is also important for protection in murine and human studies [136].
Most studies look at peripheral, systemic immune responses, but there is increasing interest in the lung environment. The pulmonary CD4+ T cell response can be divided into two subsets, one in the lung parenchyma, and one residing within the vasculature [137]. The parenchymal effectors are PD-1hi/CD69hi CD4+ T cells, which are highly proliferative, in contrast with the more terminally differentiated KLRG1hi/T-bethi CD4+ cells resident in the vasculature [137,138,139,140,141]. These KLRG1hi cells produce more IFN-ɣ [140] and are the most abundant subset in the lung at the peak of clonal expansion [137]; however, they are very poor at entering the lung parenchyma [142].
IL-17
There is also evidence that a Th17 response may be implicated in protection. IL-17 is produced principally by Th17 cells, which require both TGF-β and IL-16 for initiation [143,144,145], in addition to IL-23 in order to become an established population. These IL-17-producing cells provide a surveillance function in the periphery [146], though in excess they can are associated with excess neutrophil recruitment [147, 148] and autoimmune disease [146, 149, 150]. Pathogenic overproduction of IL-17 is restricted by IFN-ɣ [151,152,153], limiting neutrophil accumulation and coincident lung inflammation during M. tuberculosis infection. IL-17 itself drives Th1 response by overcoming IL-10 inhibition [154], thus IL-17 and IFN-ɣ have significant interplay [155]. Th17 cells have been found within the pulmonary lesions of TB patients, in addition to the less well characterised Th1/Th17 cells [156, 157]. Also known as Th1* cells, these are capable of producing both IFN-ɣ and IL-17, but their role in TB is still unclear [156].
In addition to their role in granuloma formation, there also appears to be a link between IL-17 and protective antibodies. Using the TB susceptible DBA/2 mouse strain, it was found that intranasal but not subcutaneously administered BCG conferred robust protection against pulmonary TB [158]. This was associated with an IL-17-based M. tuberculosis-specific mucosal immune response following intranasal vaccination [158]. Neutralisation of IL-17 in vivo abrogated the M. tuberculosis-specific IgA secretion seen in the respiratory airways and reduced lung expression of polymeric immunoglobulin receptor (pIgR), which translocates IgA into the airway lumen [159].
Mucosal immunity
There is increasing evidence from animal models that delivery of a vaccine direct to the respiratory mucosa may be a more protective route of vaccination. An understanding of the different T cell subsets within the lung would inform the design of vaccines targeting this route.
Lung T cell subsets
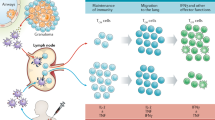
Thus far, three main subsets of lung resident memory cells have been defined: T effector memory cells (TEM), T central memory cells (TCM), and T resident memory cells (TRM). Much of the available data on this has come from murine studies. It is not always clear how these findings relate to NHPs and humans. Most of the lung resident T memory cells are of the TEM phenotype [160], CD44hi CD62Llo CD127hi [141, 161]. The TEM subset act in the first line of defence [162], predominantly secreting Th1 cytokines [160]. They are able to recirculate between blood, non-lymphoid tissues and lymph [163]. Studies of individuals with LTBI have demonstrated an increased level of the exhaustion marker PD-1 on T cells, perhaps due to continuous antigenic stimulation [164]. T cells from BCG vaccinated individuals were CD27+ but had low PD-1 expression, indicating an earlier stage of differentiation [164]. Despite this, the antigen-specific CD4+ T cell response of BCG-vaccinated human new-borns wanes over the first year of life, suggesting that the TEM population induced is unable to maintain persistent memory [165]. In response to continuous antigen exposure, TEM become terminally differentiated T effector (Teff) cells, losing the ability to proliferate and migrate into the lung parenchyma, expressing the KLRG1 marker [166, 167].
In contrast, IL-2-producing TCM have a high proliferative capacity [168], usually CD62hi CD127hi [161], and derive from KLRG1− precursors [169]. This cell population is capable of rapid proliferation, evolving into large numbers of pro-inflammatory effectors upon antigen re-exposure [168]. The lack of TCM induction by intradermal BCG may underlie the loss of protective efficacy with time [170], supported by findings that prevention of TCM exiting the lymph nodes has no influence on the protection provided by BCG [171]. This indicates that BCG promotes mainly TEM- and Teff-based responses [171].
A recent study has challenged the conventional view that TCM are necessary for vaccine-induced protection. A recombinant CMV-vectored TB vaccine achieved very high levels of protection against M. tuberculosis challenge in NHPs which was associated with the induction of TEM and transitional effector memory T cells (TTrEM), not TCM [172]. The ability of TCM to confer greater protection than TEM is possibly best shown by adoptive transfer of the separate T subsets (Kaufmann et al.), in which TCM markedly protected against TB in contrast with TEM and T follicular helper (TFH) cells [126]. These TCM cells had characteristic CXCR5+ CCR7+ expression and CXCR5 expression correlating with decreased lung pathology [126].
In mice, VPM1002 delivered subcutaneously induced a significantly increased TCM response compared with BCG, which was associated with improved protection after M. tuberculosis challenge [126]. Adoptive transfer of TCM specific for M. tuberculosis conferred protection, whereas adoptive transfer of TFH alone did not [126].
TRM are CD44hi CD62Llo CD69+ CD103+ in phenotype [141], like TCM deriving from KLRG1− precursors [169]. TRM permanently reside in non-lymphoid tissue, making them strategically placed for a rapid recall response [138]. As a group, TRM are highly heterogeneous, with some CD4+ TRM displaying a regulatory profile (Foxp3hi IL-10hi) and others with a more effector profile (T-bet+) [141]. In contrast, airway resident CD8+ TRM cells are more homogenous, expressing predominantly Th1 cytokines [141]. In addition to their cytolytic role, CD8+ TRM are also capable of activating bystander NK and B cells through IFN-ɣ, TNFα and IL-2 [173]. Maintenance of TRM may be reliant on the presence of live bacilli, as clearance of BCG in mice with chemotherapy abrogates the antigen-specific CD4+ T cell response [166]. Of all the T memory cell subtypes, the mucosal transfer of CD8+ TRM cells was associated with the most protection against M. tuberculosis challenge on a per-cell basis [141].
Despite promoting lung-localised TRM, mucosal boosting with a protein/adjuvant candidate vaccine, H56:CAF01, did not enhance protection [174]. H56 is a subunit vaccine, a fusion protein of the M. tuberculosis antigens Ag85B, ESAT-6 and Rv2660c [175], which has been combined with the liposome adjuvant CAF01. The parenteral priming followed by mucosal boosting did enhance early lung T cell response; however, mucosal boosting did not alter the cytokine profile nor conferred added protection [174]. H56:IC31 administered systemically has been evaluated in a phase 2a trial (NCT01865487) [176] and is currently recruiting for another larger scale phase 2 trial (NCT03512249).
In summary, less-differentiated CD4+ T cells seem to provide greater protection than more-differentiated effector T cells. Vaccine strategies should therefore attempt to induce these cell populations, which appear to be related to dose and persistence of the vaccine construct [165, 177].
Mucosal TB vaccines
The concept of delivering a TB vaccine direct to the respiratory mucosa is nothing new. Nebulised BCG was demonstrated to be safe and immunogenic in terms of tuberculin skin test conversion in 1968 [178]. There are concerns about intransal delivery after transient cases of facial nerve palsy following nasal subunit vaccination in two phase 1 clinical trials [179, 180]. Furthermore, there were worries that a post-exposure vaccine could trigger Koch’s phenomenon, in which reinfection is marked by rapidly developing necrotic lesions caused by hypersensitivity to the mycobacteria [181]. To date, this concerns appear unfounded, at least in BCG-primed individuals [182].
Aerosolised MVA85A, a modified Vaccinia virus Ankara expressing Ag85A, was evaluated in a proof-of-concept phase 1 trial (NCT01497769) in BCG vaccinated healthy adults [182]. In this trial, respiratory adverse events post-aerosol were rare, with no difference in occurrence compared with placebo [182]. Aerosol delivery induced more potent brochoalveolar lavage Th1 responses compared with intradermal vaccination and comparable systemic responses [182].
Adenoviruses are another promising candidate for use in a mucosal TB vaccine due to their natural tropism for respiratory epithelium [183]. Two adenovirus-based TB vaccines are AdHu5Ag85A, which has demonstrated T cell responses despite pre-existing adenoviral immunity [184], and ChAdOx1.85A [185]. Both are currently being evaluated as a nebulised vaccine (NCT02337270 and NCT04121494). An adenovirus AdHu35 expressing the M. tuberculosis antigens Ag85A, Ag5B and TB10.4, AERAS-402, had demonstrated robust cellular immune responses in the lungs of rhesus macaques, however this failed to confer added protection [186]. Whilst in mice the accumulation and retention of memory CD4+ and CD8+ T cells within the airway lumen correlated with protection against TB, this was not observed in the macaques. This was potentially due to the very large doses of M. tuberculosis used in the macaque trial [186].
Mucosal BCG vaccination has been shown to confer superior protection in the lungs compared with intradermal BCG in mice [139], and parenteral administration in guinea pigs [187] and macaques [188], associated with greater numbers of TRM and an enhanced proliferative capacity of lung parenchymal CD4+ T cells [139, 141]. The superior protection was specific to the lungs, with protection in the spleen equal to that conferred by the intradermal route [139]. CXCR3 expression, key to the recruitment of CD8+ T cells [189], was only found in lung parenchymal CD4+ T cells with mucosal BCG vaccination [139]. A recent study in NHPs has demonstrated a superior protective effect of mucosal BCG immunisation compared with intradermal immunisation against low-dose repeated M. tuberculosis infection [190]. The mucosally immunised group showed higher local levels of polyfunctional Th17 cells, IL10 and mucosal IgA [190]. Though this work is highly promising, novel methods for the immunomonitoring of aerosol vaccination are necessary, due to the invasive nature of bronchoscopy and bronchoalveolar lavage. Induced sputum is one possibility, having been used before as an immunoassay in TB patients [191, 192].
Humoral immunity
The role of humoral immunity in protection against TB was for a long time discounted. When cynomolgus macaques were treated with rituximab, a B cell depleting agent (but not plasma cell depleting), the overall disease progression and outcome of M. tuberculosis infection in the acute phase was unaltered [193]. Though no change in outcome was seen, there was a significant increase in IL-2, IL-10 and IL-17 producing T cells within rituximab treated macaques, though IL-6 and IL-10 levels were lower in the granulomas themselves [193]. IL-6 and IL-10 are both secreted by B cells [194, 195], IL-6 increasing T cell development [196] and B cell expansion [194, 197]. In B cell-deficient mice, BCG was less effective as a result of the dysregulated IL-17 production [198, 199], the elevated IL-17 resulting in greater levels of phagocytosis of BCG by neutrophils rather than monocytes [200].
By comparing the antibody profiles between those with active TB and LTBI, a functional role for antibodies is emerging. An unbiased systems serology approach found nine specific antibodies capable of distinguishing the two groups, LTBI or active TB [32]. Latent infection is associated with unique antibody glycosylation and Fc functional profiles, which drives innate immunity to kill intracellular M. tuberculosis [32]. Fc glycosylation could be a potential future biomarker, with the differences reflecting differential B cell priming [201]. Furthermore, patients with LTBI show superior NK cell mediated cytotoxicity, associated with increased levels of binding to FcɣRIII, driving NK cell activation [32, 202]. Whether these differences are a pathological mechanism of M. tuberculosis persistence or outcome of successful control are thus far unclear.
Further clues as to a role for humoral immunity come from health care workers (HCWs) with occupational exposure to M. tuberculosis [203, 204]. HCWs have slightly higher titers of M. tuberculosis-specific IgA than those with active TB, with 7/12 isolated IgA mAbs capable of restricting M. tuberculosis growth compared with 0/16 IgG mAbs [203]. In another study, no patients with active TB made protective antibody responses, whereas a subset of patients with LTBI and HCWs had antibodies capable of restricting M. tuberculosis growth [204]. This growth restriction was completely negated by the absence of CD4+ T cells, perhaps because of a requirement for immune complexes [204].
The protection shown by IgA but not IgG points to the importance of invariant antibody function in protection. M. tuberculosis infection in mice lacking activating FcɣR ɣ-chain results in more severe immunopathology during disease due to higher IL-10 levels, further supporting the importance of Ab-Fc function [205]. FcRs can be activating or inhibitory, with heterogeneity between individuals impacting whether B cells have a pro or anti-inflammatory impact at the level of the granuloma [205]. It is possible that opsonising antibodies better enable M. tuberculosis to be internalised via phagocytosis into target macrophages [203].
Though the natural protective effect of antibodies appears small, this does not necessarily mean that a humoral vaccine would fail, only that different antigens need be found. In the laboratory, M. tuberculosis is frequently grown in detergent, stripping the capsule [206]; these capsular antigens have been shown to generate IFN-ɣ and T cell responses in addition to high titers of antibody [207]. Carbohydrate-protein conjugate vaccines against arabinomannan in addition to a peptide mimotope against LAM have demonstrated efficacy in murine models [208, 209].
Correlates of protection
The lack of validated immune correlates of protection is one of the greatest challenges in TB vaccine development. Identification and validation of such correlates is possible only when samples from successful placebo controlled efficacy trials become available, requiring a comparison of the immune responses in vaccinated and unvaccinated individuals protected against M. tuberculosis in addition to those not protected [210].
The greatest potential for immune insight would be if the leading candidate TB vaccines induced a diverse immune response. However, in a recent comparison of antigen-specific T cell responses from human clinical trials, the functional profiles suggested a lack of response diversity, with the main difference in the magnitude of response [211]. This comparison involved AERAS-402, H1:IC31, M72/ASO1E, ID93+GLA-SE, H56:IC31 and MVA85A [211].
The state of the pipeline
Whole cell vaccines
Due to the difficulties in identifying antigens capable of generating a protective response, whole cell derived vaccines have gained increasing interest [212]. Whilst advantages with whole cell vaccines include a comprehensive antigen repertoire and similarity to natural infection [213], there are worries that this may simply induce a similarly semi-effectual immune response to that seen with natural M. tuberculosis infection [212].
Results reported by Nemes et al. have raised interest in the use of BCG re-vaccination, rather than simply focussing on novel vaccines [214]. This is due to a finding of 45.4% efficacy of BCG revaccination against sustained QuantiFERON TB-GOLD (QFT) conversion [214]. This was a phase IIb prevention of infection (POI) trial of H4:IC31 vs. BCG revaccination in an adolescent cohort [214]. H4 is a subunit vaccine consisting of Ag85B and TB10.4, which do not cross-react with QFT, combined with the IC31 adjuvant [214]. Though H4:IC31 induced significant increases in Ag85B and TB10.4-specific CD4+ T cell responses, neither H4:IC31 nor BCG re-vaccination prevented initial QFT conversion, failing to meet the primary endpoint [214].
Recombinant BCG strategies can broadly be divided into two camps, the first of which being those that overexpress M. tuberculosis immunodominant antigens such as rBCG30. rBCG30 overexpresses Ag85B [215] and was shown to be well tolerated and more immunogenic than BCG in a phase 1 trial [216]. The second strategy involves the modification of BCG for more effective antigen presentation and TCM induction. Some examples of BCG-based vaccines have been described throughout this review, whether they work through a return of lost virulence factors as in the case of BCG::ESX-1 [118], aiding apoptosis as achieved by BCG::BAX [217] or through aiding phagolysosome escape as seen with VPM1002 [120].
The clinical development of one recombinant BCG strain, AERAS-422, an rBCG overexpressing three mycobacterial antigens and expressing perfringolysin, was terminated after 2/8 immunised healthy volunteers developed shingles after the reactivation of varicella zoster virus [218].
Rather than creating a more immunogenic/virulent BCG, another tactic is to attenuate M. tuberculosis itself. MTBVAC has deletion of the transcription factor phoP [219], which would otherwise control intracellular adaptation of the mycobacteria and promote ESAT-6 secretion [220, 221], and deletion of fadD26, required for synthesis of virulence associated cell wall lipids (pthiocerol dimycocerosates) [222, 223]. A phase 2 trial of MTBVAC vs BCG in adults and neonates has just reported (NCT02729571), finding it to be safe and immunogenic, paving the way for larger scale trials [224].
Heat-inactivated Mycobacterium vaccae has been approved in China, yet there is little publicly available information from the Chinese trials [225], with the DarDar trial the only trial clearly showing clinical efficacy, although the primary outcome was not reached [226]. Now re-branded DAR-901, grown in broth instead of agar [212], it is currently being evaluated in a phase 2b trial (NCT02712424). Mycobacterium indicus pranii (MIP) is a non-pathogenic mycobacterium, FDA approved as a leprosy vaccine [227]. MIP has been shown to be safe in pulmonary TB patients undergoing retreatment for TB [228].
RUTI, detoxified and fragmented M. tuberculosis within liposomes, is an immunotherapeutic agent to reduce the extent and duration of required drug treatment of active TB [229] and is currently being evaluated in a phase 2a trial (NCT02711735).
Subunit vaccines
One means of retaining the protective effect of BCG is using a prime-boost strategy, in which BCG is still used, but with the addition of a heterologous vaccine booster [230]. Subunit vaccines require identification of protective antigens and also identification of an appropriate antigen delivery system which is usually a protein/adjuvant combination or a recombinant viral vector [231]. Subunit-based vaccines allow the triggering of immune memory without the safety concerns of a live vaccine, in addition to giving the short exposure that most favours TCM formation [166, 232].
The final analysis of the post-exposure phase IIb trial of the subunit vaccine M72/ASO1E has shown 50% efficacy against progression to TB relative to placebo in patients already latently infected with M. tuberculosis [233]. M72 is a fusion protein derived from the antigens M. tuberculosis32A and M. tuberculosis39A, the adjuvant also a component of the malaria vaccine RTS, S/AS01 [233]. This was an extremely important result, demonstrating the potential of novel TB vaccines in pre-sensitised populations. One key important question arising from these data are whether this vaccine would confer protection in M. tuberculosis-uninfected subjects. If not, the efficacy will be lower in areas of the world where M. tuberculosis infection prevalence is lower. Preclinical studies demonstrate some level of protection in M. tuberculosis-uninfected NHPs and guinea pigs [234, 235].
The main candidate subunit vaccines are M72/ASO1E [233] and H56:IC31 [176], previously mentioned, in addition to ID93:GLA-SE [236]. The latter is a fusion of four M. tuberculosis antigens (Rv1813, Rv2608, Rv3619 and Rv3620) [236] combined with the TLR-4 agonist adjuvant GLA-SE [237], which has completed a phase 2a trial successfully [238].
Viral-based vaccines
With their natural tropism, viral vectors allow greater targeting than subunit vaccines. The benefits of adenovirus-based vaccines have previously been discussed. TB/FLU-04l uses another respiratory epithelium tropic virus, the influenza H1N1 as a base, expressing the Ag85A and ESAT-6 antigens [227].
MVA85 was the first TB vaccine to enter efficacy trials since 1968 [7]. MVA (Modified Vaccinia Ankara) is an attenuated strain of Vaccinia virus, unable to replicate, with the addition of Ag85A [239]. Despite promoting powerful Th1 responses in early clinical trials [240], MVA85A failed to demonstrate protection in a preventative pre-exposure phase IIb trial in BCG-vaccinated infants [7].
Despite this failure, trials involving MVA85A have identified potential immune correlates [241]. In BCG-vaccinated infants, activated HLA-DR+ CD4+ T cells were associated with an increased risk of TB, a result confirmed in an adolescent cohort [241]. A linear effect was also seen with higher numbers of IFN-ɣ secreting BCG-specific T cells associated with a greater reduction in the risk of TB disease, in addition to Ag85A-specific IgG correlating with non-progression to disease [241]. This illustrates the importance of storing immune correlate samples from all efficacy trials, as these samples are valuable regardless of the efficacy result.
A novel viral vector in preclinical development as a TB vaccine candidate utilises CMV (cytomegalovirus) as the vector base [172]. Results from the subcutaneous vaccination of rhesus macaques with rhesus CMV encoding nine different M. tuberculosis antigens resulted in an overall reduction of M. tuberculosis infection by 68% compared with unvaccinated controls, with 41% negative for any disease [172]. The authors’ conclusion of sterilising immunity was based on an absence of radiological disease and negative bacterial cultures from punch biopsies [172]. CMV is highly capable of inducing TEM cells [242,243,244], though a neutrophil-specific transcriptional signature was found in vaccinated animals [172], suggesting a role for innate immunity. Further work to understand the protective mechanism and how this approach can be successfully translated to the clinic is underway.
Conclusion
Whilst the field of TB vaccine development has experienced significant hurdles, it is important to recognise the great progress made of late, both in immunological understanding and in empirical learning from human clinical trials. An immunological understanding of the pathogenesis of M. tuberculosis, one of the principal barriers to designing an effective vaccine, has slowly but surely been built up to the increasingly clear picture we have today. The classic dogma of the past, focussed solely on the adaptive response, has evolved into something far more complex, integrating the innate, adaptive and humoral systems. With this greater understanding, a variety of novel vaccine design strategies has been made possible. The recent M72/AS01E result gives renewed cause for optimism in this challenging field. It is critical to maintain the momentum that has been built up over the last two decades so that M. tuberculosis, a pathogen that has been with us for 3 million years [245], can finally be consigned to the same fate as smallpox.
References
Global Tuberculosis Report (WHO), (2019) Geneva
Keja K, Chan C, Hayden G, Henderson RH (1988) Expanded programme on immunization. World Health Stat Q 41(2):59–63
Divangahi M (2018) Are tolerance and training required to end TB? Nat Rev Immunol 18(11):661
Colditz GA, Brewer TF, Berkey CS, Wilson ME, Burdick E, Fineberg HV et al (1994) Efficacy of BCG vaccine in the prevention of tuberculosis: meta-analysis of the published literature. JAMA. 271(9):698–702
WHO, (2001). Global tuberculosis control. WHO report
Aronson NE, Santosham M, Comstock GW, Howard RS, Moulton LH, Rhoades ER et al (2004) Long-term efficacy of BCG vaccine in American Indians and Alaska natives: a 60-year follow-up study. Jama. 291(17):2086–2091
Tameris MD, Hatherill M, Landry BS, Scriba TJ, Snowden MA, Lockhart S et al (2013) Safety and efficacy of MVA85A, a new tuberculosis vaccine, in infants previously vaccinated with BCG: a randomised, placebo-controlled phase 2b trial. Lancet 381(9871):1021–1028
Tait DR, Hatherill M, Van Der Meeren O, Ginsberg AM, Van Brakel E, Salaun B et al (2019) Final analysis of a trial of M72/AS01E vaccine to prevent tuberculosis. N Engl J Med 381(25):2429–2439
Calmette A, Guérin C (1911) Recherches expérimentales sur la défense de l’organisme contre l’infection tuberculeuse. Ann Inst Pasteur 25:625–641
Camille GR, S. R. The history of BCG: early history, pp. 48–58. In: Rosenthal SR (ed). London: J&H Churchill. 1957
Hart PD, Sutherland I (1977) BCG and vole bacillus vaccines in the prevention of tuberculosis in adolescence and early adult life. Br Med J 2(6082):293–295
Comstock GW, Woolpert SF, Livesay VT (1976) Tuberculosis studies in Muscogee County, Georgia. Twenty-year evaluation of a community trial of BCG vaccination. Public Health Rep 91(3):276–280
Comstock GW, Livesay VT, Woolpert SF (1974) Evaluation of BCG vaccination among Puerto Rican children. Am J Public Health 64(3):283–291
Tuberculosis Prevention Trial, Madras (1980) Trial of BCG vaccines in South India for tuberculosis prevention. Indian J Med Res 72(Jul):1–74
Comstock GW (1994) Field trials of tuberculosis vaccines: how could we have done them better? Control Clin Trials 15(4):247–276
Calmette A.. (1931) Preventive vaccination against tuberculosis with BCG. SAGE Publications
Ritz N, Hanekom WA, Robins-Browne R, Britton WJ, Curtis N (2008) Influence of BCG vaccine strain on the immune response and protection against tuberculosis. FEMS Microbiol Rev 32(5):821–841
Brewer TF, Colditz GA (1995) Relationship between bacille Calmette-Guerin (BCG) strains and the efficacy of BCG vaccine in the prevention of tuberculosis. Clin Infect Dis 20(1):126–135
Abubakar I, Pimpin L, Ariti C, Beynon R, Mangtani P, Sterne J et al (2013) Systematic review and meta-analysis of the current evidence on the duration of protection by bacillus Calmette-Guérin vaccination against tuberculosis. Health Technol Assess (Winchester, England) 17(37):1
Falkinham JO 3rd (2009) Surrounded by mycobacteria: nontuberculous mycobacteria in the human environment. J Appl Microbiol 107(2):356–367
Burl S, Adetifa UJ, Cox M, Touray E, Ota MO, Marchant A et al (2010) Delaying bacillus Calmette-Guerin vaccination from birth to 4 1/2 months of age reduces postvaccination Th1 and IL-17 responses but leads to comparable mycobacterial responses at 9 months of age. J Immunol 185(4):2620–2628
Poyntz HC, Stylianou E, Griffiths KL, Marsay L, Checkley AM, McShane H (2014) Non-tuberculous mycobacteria have diverse effects on BCG efficacy against Mycobacterium tuberculosis. Tuberculosis (Edinb) 94(3):226–237
Black GF, Weir RE, Floyd S, Bliss L, Warndorff DK, Crampin AC et al (2002) BCG-induced increase in interferon-gamma response to mycobacterial antigens and efficacy of BCG vaccination in Malawi and the UK: two randomised controlled studies. Lancet. 359(9315):1393–1401
Brandt L, Feino Cunha J, Weinreich Olsen A, Chilima B, Hirsch P, Appelberg R et al (2002) Failure of the Mycobacterium bovis BCG vaccine: some species of environmental mycobacteria block multiplication of BCG and induction of protective immunity to tuberculosis. Infect Immun 70(2):672–678
Barreto ML, Pilger D, Pereira SM, Genser B, Cruz AA, Cunha SS et al (2014) Causes of variation in BCG vaccine efficacy: examining evidence from the BCG REVAC cluster randomized trial to explore the masking and the blocking hypotheses. Vaccine. 32(30):3759–3764
Andersen P, Doherty TM (2005) The success and failure of BCG—implications for a novel tuberculosis vaccine. Nat Rev Microbiol 3(8):656
Gupta N, Kumar R, Agrawal B (2018) New players in immunity to tuberculosis: the host microbiome, lung epithelium, and innate immune cells. Front Immunol 9:709
Meermeier EW, Lewinsohn DM (2018) Early clearance versus control: what is the meaning of a negative tuberculin skin test or interferon-gamma release assay following exposure to Mycobacterium tuberculosis? F1000Research 7:F1000 Faculty Rev-664
Mahan CS, Zalwango S, Thiel BA, Malone LL, Chervenak KA, Baseke J et al (2012) Innate and adaptive immune responses during acute M. tuberculosis infection in adult household contacts in Kampala, Uganda. Am J Trop Med Hyg 86(4):690–697
Bark CM, Manceur AM, Malone LL, Nsereko M, Okware B, Mayanja HK et al (2017) Identification of host proteins predictive of early stage Mycobacterium tuberculosis infection. EBioMedicine. 21:150–157
Reuschl A-K, Edwards MR, Parker R, Connell DW, Hoang L, Halliday A et al (2017) Innate activation of human primary epithelial cells broadens the host response to Mycobacterium tuberculosis in the airways. PLoS Pathog 13(9):e1006577
Lu LL, Chung AW, Rosebrock TR, Ghebremichael M, Yu WH, Grace PS et al (2016) A functional role for antibodies in tuberculosis. Cell. 167(2):433–43.e14
Godfrey DI, Uldrich AP, McCluskey J, Rossjohn J, Moody DB (2015) The burgeoning family of unconventional T cells. Nat Immunol 16(11):1114
Hall NB, Igo RP Jr, Malone L, Truitt B, Schnell A, Tao L et al (2015) Polymorphisms in TICAM2 and IL1B are associated with TB. Genes Immun 16(2):127
Kleinnijenhuis J, Quintin J, Preijers F, Joosten LA, Ifrim DC, Saeed S et al (2012) Bacille Calmette-Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc Natl Acad Sci 109(43):17537–17542
Agger EM (2016) Novel adjuvant formulations for delivery of anti-tuberculosis vaccine candidates. Adv Drug Deliv Rev 102:73–82
Riley R, Mills C, Nyka W, Weinstock N, Storey P, Sultan L et al (1959) Aerial dissemination of pulmonary tuberculosis. A two-year study of contagion in a tuberculosis ward. Am J Hyg 70(2):185–196
Saini D, Hopkins GW, Seay SA, Chen C-J, Perley CC, Click EM et al (2012) Ultra-low dose of Mycobacterium tuberculosis aerosol creates partial infection in mice. Tuberculosis. 92(2):160–165
Kleinnijenhuis J, Oosting M, Joosten LA, Netea MG, Van Crevel R (2011) Innate immune recognition of Mycobacterium tuberculosis. Clin Dev Immunol 2011
Ferguson J, Schlesinger L (2000) Pulmonary surfactant in innate immunity and the pathogenesis of tuberculosis. Tuber Lung Dis 80(4–5):173–184
Lee H-M, Shin D-M, Jo E-K (2009) Mycobacterium tuberculosis induces the production of tumor necrosis factor-α, interleukin-6, and CXCL8 in pulmonary epithelial cells through reactive oxygen species-dependent mitogen-activated protein kinase activation. J Bacteriol Virol 39(1):1–10
Sharma M, Sharma S, Roy S, Varma S, Bose M (2007) Pulmonary epithelial cells are a source of interferon-γ in response to Mycobacterium tuberculosis infection. Immunol Cell Biol 85(3):229–237
Lin Y, Zhang M, Barnes PF (1998) Chemokine production by a human alveolar epithelial cell line in response to Mycobacterium tuberculosis. Infect Immun 66(3):1121–1126
Nakanaga T, Nadel JA, Ueki IF, Koff JL, Shao MX (2007) Regulation of interleukin-8 via an airway epithelial signaling cascade. Am J Phys Lung Cell Mol Phys 292(5):L1289–L1L96
Harriff MJ, Cansler ME, Toren KG, Canfield ET, Kwak S, Gold MC et al (2014) Human lung epithelial cells contain Mycobacterium tuberculosis in a late endosomal vacuole and are efficiently recognized by CD8+ T cells. PLoS One 9(5):e97515
Hirsch CS, Ellner JJ, Russell DG, Rich EA (1994) Complement receptor-mediated uptake and tumor necrosis factor-alpha-mediated growth inhibition of Mycobacterium tuberculosis by human alveolar macrophages. J Immunol 152(2):743–753
Engele M, Stöβel E, Castiglione K, Schwerdtner N, Wagner M, Bölcskei P et al (2002) Induction of TNF in human alveolar macrophages as a potential evasion mechanism of virulent Mycobacterium tuberculosis. J Immunol 168(3):1328–1337
Gleeson LE, Sheedy FJ, Palsson-McDermott EM, Triglia D, O’Leary SM, O’Sullivan MP et al (2016) Cutting edge: Mycobacterium tuberculosis induces aerobic glycolysis in human alveolar macrophages that is required for control of intracellular bacillary replication. J Immunol 196(6):2444–2449
Silver RF, Walrath J, Lee H, Jacobson BA, Horton H, Bowman MR et al (2009) Human alveolar macrophage gene responses to Mycobacterium tuberculosis strains H37Ra and H37Rv. Am J Respir Cell Mol Biol 40(4):491–504
Leemans JC, Juffermans NP, Florquin S, van Rooijen N, Vervoordeldonk MJ, Verbon A et al (2001) Depletion of alveolar macrophages exerts protective effects in pulmonary tuberculosis in mice. J Immunol 166(7):4604–4611
Leemans JC, Thepen T, Weijer S, Florquin S, Van Rooijen N, Van de Winkel JG et al (2005) Macrophages play a dual role during pulmonary tuberculosis in mice. J Infect Dis 191(1):65–74
Lasco TM, Turner OC, Cassone L, Sugawara I, Yamada H, McMurray DN et al (2004) Rapid accumulation of eosinophils in lung lesions in guinea pigs infected with Mycobacterium tuberculosis. Infect Immun 72(2):1147–1149
Appelberg R (2007) Neutrophils and intracellular pathogens: beyond phagocytosis and killing. Trends Microbiol 15(2):87–92
Kibiki G, Myers L, Kalambo C, Hoang S, Stoler M, Stroup S et al (2007) Bronchoalveolar neutrophils, interferon gamma-inducible protein 10 and interleukin-7 in AIDS-associated tuberculosis. Clin Exp Immunol 148(2):254–259
Hilda JN, Narasimhan M, Das SD (2014) Neutrophils from pulmonary tuberculosis patients show augmented levels of chemokines MIP-1α, IL-8 and MCP-1 which further increase upon in vitro infection with mycobacterial strains. Hum Immunol 75(8):914–922
Petrofsky M, Bermudez LE (1999) Neutrophils from Mycobacterium avium-infected mice produce TNF-α, IL-12, and IL-1β and have a putative role in early host response. Clin Immunol 91(3):354–358
Martineau AR, Newton SM, Wilkinson KA, Kampmann B, Hall BM, Nawroly N et al (2007) Neutrophil-mediated innate immune resistance to mycobacteria. J Clin Invest 117(7):1988–1994
Goetz DH, Holmes MA, Borregaard N, Bluhm ME, Raymond KN, Strong RK (2002) The neutrophil lipocalin NGAL is a bacteriostatic agent that interferes with siderophore-mediated iron acquisition. Mol Cell 10(5):1033–1043
Dahl SL, Woodworth JS, Lerche CJ, Cramer EP, Nielsen PR, Moser C et al (2018) Lipocalin-2 functions as inhibitor of innate resistance to Mycobacterium tuberculosis. Front Immunol 9
Lowe DM, Bandara AK, Packe GE, Barker RD, Wilkinson RJ, Griffiths CJ et al (2013) Neutrophilia independently predicts death in tuberculosis. Eur Respir J 42(6):1752–1757
Berry MP, Graham CM, McNab FW, Xu Z, Bloch SA, Oni T et al (2010) An interferon-inducible neutrophil-driven blood transcriptional signature in human tuberculosis. Nature. 466(7309):973
Eruslanov EB, Lyadova IV, Kondratieva TK, Majorov KB, Scheglov IV, Orlova MO et al (2005) Neutrophil responses to Mycobacterium tuberculosis infection in genetically susceptible and resistant mice. Infect Immun 73(3):1744–1753
Allen M, Bailey C, Cahatol I, Dodge L, Yim J, Kassissa C et al (2015) Mechanisms of control of Mycobacterium tuberculosis by NK cells: role of glutathione. Front Immunol 6:508
Kee S-J, Kwon Y-S, Park Y-W, Cho Y-N, Lee S-J, Kim T-J et al (2012) Dysfunction of natural killer T cells in patients with active Mycobacterium tuberculosis infection. Infect Immun 80(6):2100–2108
Dieli F, Troye-Blomberg M, Ivanyi J, Fournié JJ, Krensky AM, Bonneville M et al (2001) Granulysin-dependent killing of intracellular and extracellular Mycobacterium tuberculosis by Vγ9/Vδ2 T lymphocytes. J Infect Dis 184(8):1082–1085
Meraviglia S, El Daker S, Dieli F, Martini F, Martino A (2011) γδ T cells cross-link innate and adaptive immunity in Mycobacterium tuberculosis infection. Clin Dev Immunol 2011
Shen Y, Zhou D, Qiu L, Lai X, Simon M, Shen L et al (2002) Adaptive immune response of Vgamma2Vdelta2+ T cells during mycobacterial infections. Science. 295(5563):2255–2258
Gold MC, Napier RJ, Lewinsohn DM (2015) MR 1-restricted mucosal associated invariant T (MAIT) cells in the immune response to M ycobacterium tuberculosis. Immunol Rev 264(1):154–166
Jiang J, Yang B, An H, Wang X, Liu Y, Cao Z et al (2016) Mucosal-associated invariant T cells from patients with tuberculosis exhibit impaired immune response. J Inf Secur 72(3):338–352
Diefenbach A, Colonna M, Koyasu S (2014) Development, differentiation, and diversity of innate lymphoid cells. Immunity. 41(3):354–365
Ardain A, Domingo-Gonzalez R, Das S, Kazer SW, Howard NC, Singh A et al (2019) Group 3 innate lymphoid cells mediate early protective immunity against tuberculosis. Nature. 1
Woodland DL, Randall TD (2004) Anatomical features of anti-viral immunity in the respiratory tract. Semin Immunol 16(3):163–170
Moyron-Quiroz JE, Rangel-Moreno J, Kusser K, Hartson L, Sprague F, Goodrich S et al (2004) Role of inducible bronchus associated lymphoid tissue (iBALT) in respiratory immunity. Nat Med 10(9):927–934
Ulrichs T, Kosmiadi GA, Trusov V, Jorg S, Pradl L, Titukhina M et al (2004) Human tuberculous granulomas induce peripheral lymphoid follicle-like structures to orchestrate local host defence in the lung. J Pathol 204(2):217–228
Ganchua SKC, Cadena AM, Maiello P, Gideon HP, Myers AJ, Junecko BF et al (2018) Lymph nodes are sites of prolonged bacterial persistence during Mycobacterium tuberculosis infection in macaques. PLoS Pathog 14(11):e1007337
Khader SA, Guglani L, Rangel-Moreno J, Gopal R, Junecko BA, Fountain JJ et al (2011) IL-23 is required for long-term control of Mycobacterium tuberculosis and B cell follicle formation in the infected lung. J Immunol 187(10):5402–5407
Slight SR, Rangel-Moreno J, Gopal R, Lin Y, Junecko BAF, Mehra S et al (2013) CXCR5+ T helper cells mediate protective immunity against tuberculosis. J Clin Invest 123(2)
Klose CS, Artis D (2016) Innate lymphoid cells as regulators of immunity, inflammation and tissue homeostasis. Nat Immunol 17(7):765
Rangel-Moreno J, Carragher DM, de la Luz Garcia-Hernandez M, Hwang JY, Kusser K, Hartson L et al (2011) The development of inducible bronchus-associated lymphoid tissue depends on IL-17. Nat Immunol 12(7):639
Joosten SA, van Meijgaarden KE, Arend SM, Prins C, Oftung F, Korsvold GE et al (2018) Mycobacterial growth inhibition is associated with trained innate immunity. J Clin Invest 128(5):1837–1851
Cros J, Cagnard N, Woollard K, Patey N, Zhang S-Y, Senechal B et al (2010) Human CD14dim monocytes patrol and sense nucleic acids and viruses via TLR7 and TLR8 receptors. Immunity. 33(3):375–386
Netea MG, Joosten LA, Latz E, Mills KH, Natoli G, Stunnenberg HG et al (2016) Trained immunity: a program of innate immune memory in health and disease. Science 352(6284):aaf1098
Higgins JP, Soares-Weiser K, López-López JA, Kakourou A, Chaplin K, Christensen H et al (2016) Association of BCG, DTP, and measles containing vaccines with childhood mortality: systematic review. BMJ. 355:i5170
Kaufmann E, Sanz J, Dunn JL, Khan N, Mendonça LE, Pacis A et al (2018) BCG educates hematopoietic stem cells to generate protective innate immunity against tuberculosis. Cell 172(1–2):176–90.e19
Orme IM, Robinson RT, Cooper AM (2015) The balance between protective and pathogenic immune responses in the TB-infected lung. Nat Immunol 16(1):57
Liu H, Moynihan KD, Zheng Y, Szeto GL, Li AV, Huang B et al (2014) Structure-based programming of lymph-node targeting in molecular vaccines. Nature. 507(7493):519
Griffiths KL, Ahmed M, Das S, Gopal R, Horne W, Connell TD et al (2016) Targeting dendritic cells to accelerate T-cell activation overcomes a bottleneck in tuberculosis vaccine efficacy. Nat Commun 7:13894
Antonelli LR, Rothfuchs AG, Gonçalves R, Roffê E, Cheever AW, Bafica A et al (2010) Intranasal poly-IC treatment exacerbates tuberculosis in mice through the pulmonary recruitment of a pathogen-permissive monocyte/macrophage population. J Clin Invest 120(5):1674–1682
Sharpe S, White A, Sarfas C, Sibley L, Gleeson F, McIntyre A et al (2016) Alternative BCG delivery strategies improve protection against Mycobacterium tuberculosis in non-human primates: protection associated with mycobacterial antigen-specific CD4 effector memory T-cell populations. Tuberculosis (Edinb) 101:174–190
Darrah PA, Zeppa JJ, Maiello P, Hackney JA, Wadsworth MH 2nd, Hughes TK et al (2020) Prevention of tuberculosis in macaques after intravenous BCG immunization. Nature. 577(7788):95–102
Master SS, Rampini SK, Davis AS, Keller C, Ehlers S, Springer B et al (2008) Mycobacterium tuberculosis prevents inflammasome activation. Cell Host Microbe 3(4):224–232
Kaushal D, Schroeder BG, Tyagi S, Yoshimatsu T, Scott C, Ko C et al (2002) Reduced immunopathology and mortality despite tissue persistence in a Mycobacterium tuberculosis mutant lacking alternative σ factor, SigH. Proc Natl Acad Sci 99(12):8330–8335
Noss EH, Pai RK, Sellati TJ, Radolf JD, Belisle J, Golenbock DT et al (2001) Toll-like receptor 2-dependent inhibition of macrophage class II MHC expression and antigen processing by 19-kDa lipoprotein of Mycobacterium tuberculosis. J Immunol 167(2):910–918
Gehring AJ, Dobos KM, Belisle JT, Harding CV, Boom WH (2004) Mycobacterium tuberculosis LprG (Rv1411c): a novel TLR-2 ligand that inhibits human macrophage class II MHC antigen processing. J Immunol 173(4):2660–2668
Pecora ND, Gehring AJ, Canaday DH, Boom WH, Harding CV (2006) Mycobacterium tuberculosis LprA is a lipoprotein agonist of TLR2 that regulates innate immunity and APC function. J Immunol 177(1):422–429
Pires D, Marques J, Pombo JP, Carmo N, Bettencourt P, Neyrolles O et al (2016) Role of cathepsins in Mycobacterium tuberculosis survival in human macrophages. Sci Rep 6:32247
Sendide K, Deghmane A-E, Pechkovsky D, Av-Gay Y, Talal A, Hmama Z (2005) Mycobacterium bovis BCG attenuates surface expression of mature class II molecules through IL-10-dependent inhibition of cathepsin S. J Immunol 175(8):5324–5332
Simeone R, Bottai D, Brosch R (2009) ESX/type VII secretion systems and their role in host–pathogen interaction. Curr Opin Microbiol 12(1):4–10
Madan-Lala R, Sia JK, King R, Adekambi T, Monin L, Khader SA et al (2014) Mycobacterium tuberculosis impairs dendritic cell functions through the serine hydrolase Hip1. J Immunol 192(9):4263–4272
Bach H, Papavinasasundaram KG, Wong D, Hmama Z, Av-Gay Y (2008) Mycobacterium tuberculosis virulence is mediated by PtpA dephosphorylation of human vacuolar protein sorting 33B. Cell Host Microbe 3(5):316–322
Sun J, Wang X, Lau A, Liao T-YA, Bucci C, Hmama Z (2010) Mycobacterial nucleoside diphosphate kinase blocks phagosome maturation in murine RAW 264.7 macrophages. PLoS One 5(1):e8769
Hinchey J, Lee S, Jeon BY, Basaraba RJ, Venkataswamy MM, Chen B et al (2007) Enhanced priming of adaptive immunity by a proapoptotic mutant of Mycobacterium tuberculosis. J Clin Invest 117(8):2279–2288
Hmama Z, Peña-Díaz S, Joseph S, Av-Gay Y (2015) Immunoevasion and immunosuppression of the macrophage by M ycobacterium tuberculosis. Immunol Rev 264(1):220–232
Schaible UE, Winau F, Sieling PA, Fischer K, Collins HL, Hagens K et al (2003) Apoptosis facilitates antigen presentation to T lymphocytes through MHC-I and CD1 in tuberculosis. Nat Med 9(8):1039
Johansen P, Fettelschoss A, Amstutz B, Selchow P, Waeckerle-Men Y, Keller P et al (2011) Relief from Zmp1-mediated arrest of phagosome maturation is associated with facilitated presentation and enhanced immunogenicity of mycobacterial antigens. Clin Vaccine Immunol 18(6):907–913
Sander P, Clark S, Petrera A, Vilaplana C, Meuli M, Selchow P et al (2015) Deletion of zmp1 improves Mycobacterium bovis BCG-mediated protection in a guinea pig model of tuberculosis. Vaccine. 33(11):1353–1359
Khatri B, Whelan A, Clifford D, Petrera A, Sander P, Vordermeier HM (2014) BCG Δzmp1 vaccine induces enhanced antigen specific immune responses in cattle. Vaccine. 32(7):779–784
Fujita M, Harada E, Matsumoto T, Mizuta Y, Ikegame S, Ouchi H et al (2010) Impaired host defence against Mycobacterium avium in mice with chronic granulomatous disease. Clin Exp Immunol 160(3):457–460
Fleury C, Mignotte B, Vayssière J-L (2002) Mitochondrial reactive oxygen species in cell death signaling. Biochimie. 84(2–3):131–141
Miller JL, Velmurugan K, Cowan MJ, Briken V (2010) The type I NADH dehydrogenase of Mycobacterium tuberculosis counters phagosomal NOX2 activity to inhibit TNF-α-mediated host cell apoptosis. PLoS Pathog 6(4):e1000864
Gengenbacher M, Nieuwenhuizen N, Vogelzang A, Liu H, Kaiser P, Schuerer S et al (2016) Deletion of nuoG from the vaccine candidate Mycobacterium bovis BCG ΔureC::hly improves protection against tuberculosis. MBio. 7(3):e00679–e00616
Kernodle DS (2010) Decrease in the effectiveness of bacille Calmette-Guérin vaccine against pulmonary tuberculosis: a consequence of increased immune suppression by microbial antioxidants, not overattenuation. Clin Infect Dis 51(2):177–184
Kaushal D, Foreman TW, Gautam US, Alvarez X, Adekambi T, Rangel-Moreno J et al (2015) Mucosal vaccination with attenuated Mycobacterium tuberculosis induces strong central memory responses and protects against tuberculosis. Nat Commun 6:8533
Upadhyay S, Mittal E, Philips J (2018) Tuberculosis and the art of macrophage manipulation. Pathogens Disease 76(4):fty037
Soualhine H, Deghmane A-E, Sun J, Mak K, Talal A, Av-Gay Y et al (2007) Mycobacterium bovis bacillus Calmette-Guerin secreting active cathepsin S stimulates expression of mature MHC class II molecules and antigen presentation in human macrophages. J Immunol 179(8):5137–5145
Mahairas GG, Sabo PJ, Hickey MJ, Singh DC, Stover CK (1996) Molecular analysis of genetic differences between Mycobacterium bovis BCG and virulent M. bovis. J Bacteriol 178(5):1274–1282
Romagnoli A, Etna MP, Giacomini E, Pardini M, Remoli ME, Corazzari M et al (2012) ESX-1 dependent impairment of autophagic flux by Mycobacterium tuberculosis in human dendritic cells. Autophagy. 8(9):1357–1370
Pym AS, Brodin P, Majlessi L, Brosch R, Demangel C, Williams A et al (2003) Recombinant BCG exporting ESAT-6 confers enhanced protection against tuberculosis. Nat Med 9(5):533
Gröschel MI, Sayes F, Shin SJ, Frigui W, Pawlik A, Orgeur M et al (2017) Recombinant BCG expressing ESX-1 of Mycobacterium marinum combines low virulence with cytosolic immune signaling and improved TB protection. Cell Rep 18(11):2752–2765
Hess J, Miko D, Catic A, Lehmensiek V, Russell DG, Kaufmann SH (1998) Mycobacterium bovis bacille Calmette–Guérin strains secreting listeriolysin of Listeria monocytogenes. Proc Natl Acad Sci 95(9):5299–5304
Grode L, Seiler P, Baumann S, Hess J, Brinkmann V, Eddine AN et al (2005) Increased vaccine efficacy against tuberculosis of recombinant Mycobacterium bovis bacille Calmette-Guerin mutants that secrete listeriolysin. J Clin Invest 115(9):2472–2479
Hamon MA, Ribet D, Stavru F, Cossart P (2012) Listeriolysin O: the Swiss army knife of Listeria. Trends Microbiol 20(8):360–368
Shaughnessy LM, Hoppe AD, Christensen KA, Swanson JA (2006) Membrane perforations inhibit lysosome fusion by altering pH and calcium in Listeria monocytogenes vacuoles. Cell Microbiol 8(5):781–792
Geoffroy C, Gaillard J-L, Alouf JE, Berche P (1987) Purification, characterization, and toxicity of the sulfhydryl-activated hemolysin listeriolysin O from Listeria monocytogenes. Infect Immun 55(7):1641–1646
Nieuwenhuizen NE, Kulkarni PS, Shaligram U, Cotton MF, Rentsch CA, Eisele B et al (2017) The recombinant bacille Calmette–Guérin vaccine VPM1002: ready for clinical efficacy testing. Front Immunol 8:1147
Vogelzang A, Perdomo C, Zedler U, Kuhlmann S, Hurwitz R, Gengenbacher M et al (2014) Central memory CD4+ T cells are responsible for the recombinant Bacillus Calmette-Guérin Δ ureC::hly vaccine’s superior protection against tuberculosis. J Infect Dis 210(12):1928–1937
Kaufmann SH, Cotton MF, Eisele B, Gengenbacher M, Grode L, Hesseling AC et al (2014) The BCG replacement vaccine VPM1002: from drawing board to clinical trial. Expert Rev Vaccines 13(5):619–630
Velmurugan K, Grode L, Chang R, Fitzpatrick M, Laddy D, Hokey D et al (2013) Nonclinical development of BCG replacement vaccine candidates. Vaccines. 1(2):120–138
Grode L, Ganoza CA, Brohm C, Weiner J 3rd, Eisele B, Kaufmann SH (2013) Safety and immunogenicity of the recombinant BCG vaccine VPM1002 in a phase 1 open-label randomized clinical trial. Vaccine. 31(9):1340–1348
Loxton AG, Knaul JK, Grode L, Gutschmidt A, Meller C, Eisele B et al (2017) Safety and immunogenicity of the recombinant Mycobacterium bovis BCG vaccine VPM1002 in HIV-unexposed newborn infants in South Africa. Clin Vaccine Immunol 24(2):e00439–e00416
Flynn JL, Chan J, Triebold KJ, Dalton DK, Stewart TA, Bloom BR (1993) An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med 178(6):2249–2254
Cooper AM, Dalton DK, Stewart TA, Griffin JP, Russell DG, Orme IM (1993) Disseminated tuberculosis in interferon gamma gene-disrupted mice. J Exp Med 178(6):2243–2247
Pawlowski A, Jansson M, Sköld M, Rottenberg ME, Källenius G (2012) Tuberculosis and HIV co-infection. PLoS Pathog 8(2):e1002464
Lin PL, Rutledge T, Green AM, Bigbee M, Fuhrman C, Klein E et al (2012) CD4 T cell depletion exacerbates acute Mycobacterium tuberculosis while reactivation of latent infection is dependent on severity of tissue depletion in cynomolgus macaques. AIDS Res Hum Retrovir 28(12):1693–1702
Filipe-Santos O, Bustamante J, Chapgier A, Vogt G, de Beaucoudrey L, Feinberg J et al (2006;Elsevier) Inborn errors of IL-12/23-and IFN-γ-mediated immunity: molecular, cellular, and clinical features. Semin Immunol 18(6):347–361
Zeng G, Zhang G, Chen X (2018) Th1 cytokines, true functional signatures for protective immunity against TB? Cell Mol Immunol 15(3):206
Sakai S, Kauffman KD, Schenkel JM, McBerry CC, Mayer-Barber KD, Masopust D et al (2014) Cutting edge: control of Mycobacterium tuberculosis infection by a subset of lung parenchyma–homing CD4 T cells. J Immunol 192(7):2965–2969
Gebhardt T, Wakim LM, Eidsmo L, Reading PC, Heath WR, Carbone FR (2009) Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat Immunol 10(5):524
Bull N, Stylianou E, Kaveh D, Pinpathomrat N, Pasricha J, Harrington-Kandt R et al (2019) Enhanced protection conferred by mucosal BCG vaccination associates with presence of antigen-specific lung tissue-resident PD-1+ KLRG1− CD4+ T cells. Mucosal Immunol 12(2):555
Sallin MA, Sakai S, Kauffman KD, Young HA, Zhu J, Barber DL (2017) Th1 differentiation drives the accumulation of intravascular, non-protective CD4 T cells during tuberculosis. Cell Rep 18(13):3091–3104
Perdomo C, Zedler U, Kühl AA, Lozza L, Saikali P, Sander LE et al (2016) Mucosal BCG vaccination induces protective lung-resident memory T cell populations against tuberculosis. MBio. 7(6):e01686–e01616
Kauffman KD, Sallin MA, Sakai S, Kamenyeva O, Kabat J, Weiner D et al (2018) Defective positioning in granulomas but not lung-homing limits CD4 T-cell interactions with Mycobacterium tuberculosis-infected macrophages in rhesus macaques. Mucosal Immunol 11(2):462
Mangan PR, Harrington LE, O'Quinn DB, Helms WS, Bullard DC, Elson CO et al (2006) Transforming growth factor-β induces development of the T H 17 lineage. Nature. 441(7090):231
Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B (2006) TGFβ in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 24(2):179–189
Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M et al (2006) Reciprocal developmental pathways for the generation of pathogenic effector T H 17 and regulatory T cells. Nature. 441(7090):235
Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD et al (2005) IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med 201(2):233–240
Ye P, Rodriguez FH, Kanaly S, Stocking KL, Schurr J, Schwarzenberger P et al (2001) Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med 194(4):519–528
Fujiwara M, Hirose K, Kagami S-I, Takatori H, Wakashin H, Tamachi T et al (2007) T-bet inhibits both TH2 cell-mediated eosinophil recruitment and TH17 cell-mediated neutrophil recruitment into the airways. J Allergy Clin Immunol 119(3):662–670
Nakae S, Saijo S, Horai R, Sudo K, Mori S, Iwakura Y (2003) IL-17 production from activated T cells is required for the spontaneous development of destructive arthritis in mice deficient in IL-1 receptor antagonist. Proc Natl Acad Sci 100(10):5986–5990
Nakae S, Nambu A, Sudo K, Iwakura Y (2003) Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. J Immunol 171(11):6173–6177
Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang Y-H et al (2005) A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol 6(11):1133
Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM et al (2005) Interleukin 17–producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol 6(11):1123
Cruz A, Khader SA, Torrado E, Fraga A, Pearl JE, Pedrosa J et al (2006) Cutting edge: IFN-γ regulates the induction and expansion of IL-17-producing CD4 T cells during mycobacterial infection. J Immunol 177(3):1416–1420
Gopal R, Lin Y, Obermajer N, Slight S, Nuthalapati N, Ahmed M et al (2012) IL-23-dependent IL-17 drives Th1-cell responses following Mycobacterium bovis BCG vaccination. Eur J Immunol 42(2):364–373
Khader SA, Bell GK, Pearl JE, Fountain JJ, Rangel-Moreno J, Cilley GE et al (2007) IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat Immunol 8(4):369
Nikitina IY, Panteleev AV, Kosmiadi GA, Serdyuk YV, Nenasheva TA, Nikolaev AA et al (2018) Th1, Th17, and Th1Th17 lymphocytes during tuberculosis: Th1 lymphocytes predominate and appear as low-differentiated CXCR3+ CCR6+ cells in the blood and highly differentiated CXCR3+/− CCR6− cells in the lungs. J Immunol 200(6):2090–2103
Acosta-Rodriguez EV, Rivino L, Geginat J, Jarrossay D, Gattorno M, Lanzavecchia A et al (2007) Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol 8(6):639
Aguilo N, Alvarez-Arguedas S, Uranga S, Marinova D, Monzón M, Badiola J et al (2015) Pulmonary but not subcutaneous delivery of BCG vaccine confers protection to tuberculosis-susceptible mice by an interleukin 17–dependent mechanism. J Infect Dis 213(5):831–839
Jaffar Z, Ferrini ME, Herritt LA, Roberts K (2009) Cutting edge: lung mucosal Th17-mediated responses induce polymeric Ig receptor expression by the airway epithelium and elevate secretory IgA levels. J Immunol 182(8):4507–4511
Purwar R, Campbell J, Murphy G, Richards WG, Clark RA, Kupper TS (2011) Resident memory T cells (TRM) are abundant in human lung: diversity, function, and antigen specificity. PLoS One 6(1):e16245
Huster KM, Busch V, Schiemann M, Linkemann K, Kerksiek KM, Wagner H et al (2004) Selective expression of IL-7 receptor on memory T cells identifies early CD40L-dependent generation of distinct CD8+ memory T cell subsets. Proc Natl Acad Sci 101(15):5610–5615
Henao-Tamayo M, Ordway DJ, Orme IM (2014) Memory T cell subsets in tuberculosis: what should we be targeting? Tuberculosis. 94(5):455–461
Sallusto F, Lenig D, Förster R, Lipp M, Lanzavecchia A (1999) Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 401(6754):708
Adekambi T, Ibegbu CC, Kalokhe AS, Yu T, Ray SM, Rengarajan J (2012) Distinct effector memory CD4+ T cell signatures in latent Mycobacterium tuberculosis infection, BCG vaccination and clinically resolved tuberculosis. PLoS One 7(4):e36046
Soares AP, Kwong Chung CK, Choice T, Hughes EJ, Jacobs G, van Rensburg EJ et al (2013) Longitudinal changes in CD4+ T-cell memory responses induced by BCG vaccination of newborns. J Infect Dis 207(7):1084–1094
Kaveh DA, Garcia-Pelayo MC, Hogarth PJ (2014) Persistent BCG bacilli perpetuate CD4 T effector memory and optimal protection against tuberculosis. Vaccine. 32(51):6911–6918
Schenkel JM, Masopust D (2014) Tissue-resident memory T cells. Immunity. 41(6):886–897
Geginat J, Lanzavecchia A, Sallusto F (2003) Proliferation and differentiation potential of human CD8+ memory T-cell subsets in response to antigen or homeostatic cytokines. Blood. 101(11):4260–4266
Obar JJ, Lefrançois L (2010) Early signals during CD8+ T cell priming regulate the generation of central memory cells. J Immunol 185(1):263–272
Orme IM (2010) The Achilles heel of BCG. Tuberculosis. 90(6):329–332
Connor LM, Harvie MC, Rich FJ, Quinn KM, Brinkmann V, Gros GL et al (2010) A key role for lung-resident memory lymphocytes in protective immune responses after BCG vaccination. Eur J Immunol 40(9):2482–2492
Hansen SG, Zak DE, Xu G, Ford JC, Marshall EE, Malouli D et al (2018) Prevention of tuberculosis in rhesus macaques by a cytomegalovirus-based vaccine. Nat Med 24(2):130–143
Schenkel JM, Fraser KA, Beura LK, Pauken KE, Vezys V, Masopust D (2014) Resident memory CD8 T cells trigger protective innate and adaptive immune responses. Science. 346(6205):98–101
Woodworth JS, Christensen D, Cassidy JP, Agger EM, Mortensen R, Andersen P (2019) Mucosal boosting of H56: CAF01 immunization promotes lung-localized T cells and an accelerated pulmonary response to Mycobacterium tuberculosis infection without enhancing vaccine protection. Mucosal Immunol 12(3):816
Aagaard C, Hoang T, Dietrich J, Cardona P-J, Izzo A, Dolganov G et al (2011) A multistage tuberculosis vaccine that confers efficient protection before and after exposure. Nat Med 17(2):189
Suliman S, Luabeya AKK, Geldenhuys H, Tameris M, Hoff ST, Shi Z et al (2019) Dose optimization of H56: IC31 vaccine for tuberculosis-endemic populations. A double-blind, placebo-controlled, dose-selection trial. Am J Respir Crit Care Med 199(2):220–231
Aagaard C, Hoang TTKT, Izzo A, Billeskov R, Troudt J, Arnett K et al (2009) Protection and polyfunctional T cells induced by Ag85B-TB10. 4/IC31® against Mycobacterium tuberculosis is highly dependent on the antigen dose. PLoS One 4(6):e5930
Rosenthal SR, McEnery JT, Raisys N (1968) Aerogenic BCG vaccination against tuberculosis in animal and human subjects. J Asthma Res 5(4):309–323
Mutsch M, Zhou W, Rhodes P, Bopp M, Chen RT, Linder T et al (2004) Use of the inactivated intranasal influenza vaccine and the risk of Bell's palsy in Switzerland. N Engl J Med 350(9):896–903
Lewis DJ, Huo Z, Barnett S, Kromann I, Giemza R, Galiza E et al (2009) Transient facial nerve paralysis (Bell’s palsy) following intranasal delivery of a genetically detoxified mutant of Escherichia coli heat labile toxin. PLoS One 4(9):e6999
Koch R (1891) A further communication on a remedy for tuberculosis. Br Med J 1(1568):125
Satti I, Meyer J, Harris SA, Thomas Z-RM, Griffiths K, Antrobus RD et al (2014) Safety and immunogenicity of a candidate tuberculosis vaccine MVA85A delivered by aerosol in BCG-vaccinated healthy adults: a phase 1, double-blind, randomised controlled trial. Lancet Infect Dis 14(10):939–946
Arnberg N (2009) Adenovirus receptors: implications for tropism, treatment and targeting. Rev Med Virol 19(3):165–178
Smaill F, Jeyanathan M, Smieja M, Medina MF, Thanthrige-Don N, Zganiacz A et al (2013) A human type 5 adenovirus-based tuberculosis vaccine induces robust T cell responses in humans despite preexisting anti-adenovirus immunity. Sci Transl Med 5(205):205ra134–205ra134
Wilkie M, Satti I, Minhinnick A, Harris S, Riste M, Ramon RL et al (2020) A phase I trial evaluating the safety and immunogenicity of a candidate tuberculosis vaccination regimen, ChAdOx1 85A prime—MVA85A boost in healthy UK adults. Vaccine. 38(4):779–789
Darrah PA, Bolton DL, Lackner AA, Kaushal D, Aye PP, Mehra S et al (2014) Aerosol vaccination with AERAS-402 elicits robust cellular immune responses in the lungs of rhesus macaques but fails to protect against high-dose Mycobacterium tuberculosis challenge. J Immunol 193(4):1799–1811
Garcia-Contreras L, Wong Y-L, Muttil P, Padilla D, Sadoff J, DeRousse J et al (2008) Immunization by a bacterial aerosol. Proc Natl Acad Sci 105(12):4656–4660
Barclay WR, Busey WM, Dalgard DW, Good RC, Janicki BW, Kasik JE et al (1973) Protection of monkeys against airborne tuberculosis by aerosol vaccination with bacillus Calmette-Guerin. Am Rev Respir Dis 107(3):351–358
Kohlmeier JE, Reiley WW, Perona-Wright G, Freeman ML, Yager EJ, Connor LM et al (2011) Inflammatory chemokine receptors regulate CD8+ T cell contraction and memory generation following infection. J Exp Med 208(8):1621–1634
Dijkman K, Sombroek CC, Vervenne RAW, Hofman SO, Boot C, Remarque EJ et al (2019) Prevention of tuberculosis infection and disease by local BCG in repeatedly exposed rhesus macaques. Nat Med 25(2):255–262
Brown M, Varia H, Bassett P, Davidson RN, Wall R, Pasvol G (2007) Prospective study of sputum induction, gastric washing, and bronchoalveolar lavage for the diagnosis of pulmonary tuberculosis in patients who are unable to expectorate. Clin Infect Dis 44(11):1415–1420
Manjaly Thomas Z-R, McShane H (2015) Aerosol immunisation for TB: matching route of vaccination to route of infection. Trans R Soc Trop Med Hyg 109(3):175–181
Phuah J, Wong EA, Gideon HP, Maiello P, Coleman MT, Hendricks MR et al (2016) Effects of B cell depletion on early Mycobacterium tuberculosis infection in cynomolgus macaques. Infect Immun 84(5):1301–1311
Barr TA, Shen P, Brown S, Lampropoulou V, Roch T, Lawrie S et al (2012) B cell depletion therapy ameliorates autoimmune disease through ablation of IL-6-producing B cells. J Exp Med 209(5):1001–1010
Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM (2002) B cells regulate autoimmunity by provision of IL-10. Nat Immunol 3(10):944
McGeachy MJ, Bak-Jensen KS, Chen Y, Tato CM, Blumenschein W, McClanahan T et al (2007) TGF-β and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T H-17 cell-mediated pathology. Nat Immunol 8(12):1390
Ireland SJ, Monson NL, Davis LS (2015) Seeking balance: potentiation and inhibition of multiple sclerosis autoimmune responses by IL-6 and IL-10. Cytokine. 73(2):236–244
Nikonenko B, Apt A, Mezhlumova M, Avdienko V, Yeremeev V, Moroz A (1996) Influence of the mouse BcgTbc-1 and xid genes on resistance and immune responses to tuberculosis infection and efficacy of bacille Calmette–Guérin (BCG) vaccination. Clin Exp Immunol 104(1):37–43
Kozakiewicz L, Chen Y, Xu J, Wang Y, Dunussi-Joannopoulos K, Ou Q et al (2013) B cells regulate neutrophilia during Mycobacterium tuberculosis infection and BCG vaccination by modulating the interleukin-17 response. PLoS Pathog 9(7):e1003472
Kondratieva TK, Rubakova EI, Linge IA, Evstifeev VV, Majorov KB, Apt AS (2010) B cells delay neutrophil migration toward the site of stimulus: tardiness critical for effective bacillus Calmette-Guérin vaccination against tuberculosis infection in mice. J Immunol 184(3):1227–1234
Mahan AE, Jennewein MF, Suscovich T, Dionne K, Tedesco J, Chung AW et al (2016) Antigen-specific antibody glycosylation is regulated via vaccination. PLoS Pathog 12(3):e1005456
Ravetch JV, Perussia B (1989) Alternative membrane forms of Fc gamma RIII (CD16) on human natural killer cells and neutrophils. Cell type-specific expression of two genes that differ in single nucleotide substitutions. J Exp Med 170(2):481–497
Zimmermann N, Thormann V, Hu B, Köhler AB, Imai-Matsushima A, Locht C et al (2016) Human isotype-dependent inhibitory antibody responses against Mycobacterium tuberculosis. EMBO Molecular Medicine 8(11):1325–1339
Li H, Wang X-X, Wang B, Fu L, Liu G, Lu Y et al (2017) Latently and uninfected healthcare workers exposed to TB make protective antibodies against Mycobacterium tuberculosis. Proc Natl Acad Sci 114(19):5023–5028
Maglione PJ, Xu J, Casadevall A, Chan J (2008) Fcγ receptors regulate immune activation and susceptibility during Mycobacterium tuberculosis infection. J Immunol 180(5):3329–3338
Sani M, Houben EN, Geurtsen J, Pierson J, De Punder K, van Zon M et al (2010) Direct visualization by cryo-EM of the mycobacterial capsular layer: a labile structure containing ESX-1-secreted proteins. PLoS Pathog 6(3):e1000794
Prados-Rosales R, Carreño LJ, Weinrick B, Batista-Gonzalez A, Glatman-Freedman A, Xu J et al (2016) The type of growth medium affects the presence of a mycobacterial capsule and is associated with differences in protective efficacy of BCG vaccination against Mycobacterium tuberculosis. J Infect Dis 214(3):426–437
Prados-Rosales R, Carreño L, Cheng T, Blanc C, Weinrick B, Malek A et al (2017) Enhanced control of Mycobacterium tuberculosis extrapulmonary dissemination in mice by an arabinomannan-protein conjugate vaccine. PLoS Pathog 13(3):e1006250
Shin H-J, Franco LH, Nair VR, Collins AC, Shiloh MU (2017) A baculovirus-conjugated mimotope vaccine targeting Mycobacterium tuberculosis lipoarabinomannan. PLoS One 12(10):e0185945
Qin L, Gilbert PB, Corey L, McElrath MJ, Self SG (2007) A framework for assessing immunological correlates of protection in vaccine trials. J Infect Dis 196(9):1304–1312
Rodo MJ, Rozot V, Nemes E, Dintwe O, Hatherill M, Little F et al (2019) A comparison of antigen-specific T cell responses induced by six novel tuberculosis vaccine candidates. PLoS Pathog 15(3):e1007643
Fletcher HA, Schrager L (2016) TB vaccine development and the end TB strategy: importance and current status. Trans R Soc Trop Med Hyg 110(4):212–218
Zhu B, Dockrell HM, Ottenhoff TH, Evans TG, Zhang Y (2018) Tuberculosis vaccines: opportunities and challenges. Respirology. 23(4):359–368
Nemes E, Geldenhuys H, Rozot V, Rutkowski KT, Ratangee F, Bilek N et al (2018) Prevention of M. tuberculosis infection with H4: IC31 vaccine or BCG revaccination. N Engl J Med 379(2):138–149
Horwitz MA, Harth G (2003) A new vaccine against tuberculosis affords greater survival after challenge than the current vaccine in the guinea pig model of pulmonary tuberculosis. Infect Immun 71(4):1672–1679
Hoft DF, Blazevic A, Abate G, Hanekom WA, Kaplan G, Soler JH et al (2008) A new recombinant bacille Calmette-Guerin vaccine safely induces significantly enhanced tuberculosis-specific immunity in human volunteers. J Infect Dis 198(10):1491–1501
Li G, Liu G, Song N, Kong C, Huang Q, Su H et al (2015) A novel recombinant BCG-expressing pro-apoptotic protein BAX enhances Th1 protective immune responses in mice. Mol Immunol 66(2):346–356
Hoft DF, Blazevic A, Selimovic A, Turan A, Tennant J, Abate G et al (2016) Safety and immunogenicity of the recombinant BCG vaccine AERAS-422 in healthy BCG-naïve adults: a randomized, active-controlled, first-in-human phase 1 trial. EBioMedicine. 7:278–286
Arbues A, Aguilo JI, Gonzalo-Asensio J, Marinova D, Uranga S, Puentes E et al (2013) Construction, characterization and preclinical evaluation of MTBVAC, the first live-attenuated M. tuberculosis-based vaccine to enter clinical trials. Vaccine. 31(42):4867–4873
Frigui W, Bottai D, Majlessi L, Monot M, Josselin E, Brodin P et al (2008) Control of M. tuberculosis ESAT-6 secretion and specific T cell recognition by PhoP. PLoS Pathog 4(2):e33
Walters SB, Dubnau E, Kolesnikova I, Laval F, Daffe M, Smith I (2006) The Mycobacterium tuberculosis PhoPR two-component system regulates genes essential for virulence and complex lipid biosynthesis. Mol Microbiol 60(2):312–330
Camacho LR, Ensergueix D, Perez E, Gicquel B, Guilhot C (1999) Identification of a virulence gene cluster of Mycobacterium tuberculosis by signature-tagged transposon mutagenesis. Mol Microbiol 34(2):257–267
Cox JS, Chen B, McNeil M, Jacobs WR Jr (1999) Complex lipid determines tissue-specific replication of Mycobacterium tuberculosis in mice. Nature. 402(6757):79
Tameris M, Mearns H, Penn-Nicholson A, Gregg Y, Bilek N, Mabwe S et al (2019) Live-attenuated Mycobacterium tuberculosis vaccine MTBVAC versus BCG in adults and neonates: a randomised controlled, double-blind dose-escalation trial. Lancet Respir Med 7(9):757–770
Butov DA, Pashkov YN, Stepanenko AL, Choporova AI, Butova TS, Batdelger D et al (2011) Phase IIb randomized trial of adjunct immunotherapy in patients with first-diagnosed tuberculosis, relapsed and multi-drug-resistant (MDR) TB. J Immune Based Ther Vaccines 9(1):3
von Reyn CF, Mtei L, Arbeit RD, Waddell R, Cole B, Mackenzie T et al (2010) Prevention of tuberculosis in bacille Calmette–Guérin-primed, HIV-infected adults boosted with an inactivated whole-cell mycobacterial vaccine. Aids. 24(5):675–685
Soundarya J, Ranganathan UD, Tripathy SP (2019) Current trends in tuberculosis vaccine. Med J Armed Forces India 75(1):18–24
Sharma SK, Katoch K, Sarin R, Balambal R, Jain NK, Patel N et al (2017) Efficacy and safety of Mycobacterium indicus pranii as an adjunct therapy in category II pulmonary tuberculosis in a randomized trial. Sci Rep 7(1):3354
Cardona P-J (2006) RUTI: a new chance to shorten the treatment of latent tuberculosis infection. Tuberculosis. 86(3–4):273–289
McShane H, Hill A (2005) Prime-boost immunisation strategies for tuberculosis. Microbes Infect 7(5–6):962–967
Andersen P, Woodworth JS (2014) Tuberculosis vaccines—rethinking the current paradigm. Trends Immunol 35(8):387–395
Lindenstrøm T, Agger EM, Korsholm KS, Darrah PA, Aagaard C, Seder RA et al (2009) Tuberculosis subunit vaccination provides long-term protective immunity characterized by multifunctional CD4 memory T cells. J Immunol 182(12):8047–8055
Van Der Meeren O, Hatherill M, Nduba V, Wilkinson RJ, Muyoyeta M, Van Brakel E et al (2018) Phase 2b controlled trial of M72/AS01E vaccine to prevent tuberculosis. N Engl J Med 379(17):1621–1634
Reed SG, Coler RN, Dalemans W, Tan EV, DeLa Cruz EC, Basaraba RJ et al (2009) Defined tuberculosis vaccine, Mtb72F/AS02A, evidence of protection in cynomolgus monkeys. Proc Natl Acad Sci U S A 106(7):2301–2306
Brandt L, Skeiky YA, Alderson MR, Lobet Y, Dalemans W, Turner OC et al (2004) The protective effect of the Mycobacterium bovis BCG vaccine is increased by coadministration with the Mycobacterium tuberculosis 72-kilodalton fusion polyprotein Mtb72F in M. tuberculosis-infected guinea pigs. Infect Immun 72(11):6622–6632
Bertholet S, Ireton GC, Ordway DJ, Windish HP, Pine SO, Kahn M et al (2010) A defined tuberculosis vaccine candidate boosts BCG and protects against multidrug-resistant Mycobacterium tuberculosis. Sci Transl Med 2(53):53ra74–53ra74
Anderson RC, Fox CB, Dutill TS, Shaverdian N, Evers TL, Poshusta GR et al (2010) Physicochemical characterization and biological activity of synthetic TLR4 agonist formulations. Colloids Surf B: Biointerfaces 75(1):123–132
Penn-Nicholson A, Tameris M, Smit E, Day TA, Musvosvi M, Jayashankar L et al (2018) Safety and immunogenicity of the novel tuberculosis vaccine ID93+ GLA-SE in BCG-vaccinated healthy adults in South Africa: a randomised, double-blind, placebo-controlled phase 1 trial. Lancet Respir Med 6(4):287–298
McShane H, Pathan AA, Sander CR, Keating SM, Gilbert SC, Huygen K et al (2004) Recombinant modified vaccinia virus Ankara expressing antigen 85A boosts BCG-primed and naturally acquired antimycobacterial immunity in humans. Nat Med 10(11):1240
Scriba TJ, Tameris M, Mansoor N, Smit E, van der Merwe L, Mauff K et al (2011) Dose-finding study of the novel tuberculosis vaccine, MVA85A, in healthy BCG-vaccinated infants. J Infect Dis 203(12):1832–1843
Fletcher HA, Snowden MA, Landry B, Rida W, Satti I, Harris SA et al (2016) T-cell activation is an immune correlate of risk in BCG vaccinated infants. Nat Commun 7:11290
Čičin-Šain L, Sylwester AW, Hagen SI, Siess DC, Currier N, Legasse AW et al (2011) Cytomegalovirus-specific T cell immunity is maintained in immunosenescent rhesus macaques. J Immunol 187(4):1722–1732
Jarvis MA, Hansen SG, Nelson JA, Picker LJ, Fruh K (2013) Vaccine vectors using the unique biology and immunology of cytomegalovirus. Cytomegaloviruses: from Molecular Pathogenesis to Intervention 2:450–463
Hansen SG, Wu HL, Burwitz BJ, Hughes CM, Hammond KB, Ventura AB et al (2016) Broadly targeted CD8+ T cell responses restricted by major histocompatibility complex E. Science. 351(6274):714–720
Gutierrez MC, Brisse S, Brosch R, Fabre M, Omaïs B, Marmiesse M et al (2005) Ancient origin and gene mosaicism of the progenitor of Mycobacterium tuberculosis. PLoS Pathog 1(1):e5
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is a contribution to the special issue on: Immunopathology of unresolved tropical diseases - Guest Editor: Marcel Tanner
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.