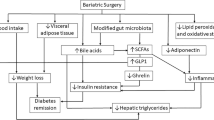
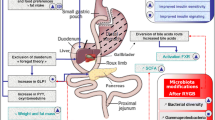
Abstract
Obesity is a chronic low-grade inflammatory disease (both at the systemic and adipose tissue level) that continues to rise worldwide. It is associated with an abundance of comorbidities, including type 2 diabetes (T2D). Bariatric surgery, which induces modifications of the intestinal tract, is to date the most successful treatment for obesity. Its use has dramatically increased in number as it enables both weight reduction and metabolic improvements, with 60% of patients even achieving diabetes remission. Several mechanisms are actually demonstrated to be involved in those clinical improvements. Importantly, both obesity and T2D share many phenotypic characteristics, including increased systemic and adipose tissue inflammation, as well as gut microbiota dysbiosis. These characteristics are deeply modulated after bariatric surgery. This review will address the host metabolic changes observed after bariatric surgery, focusing on the induced gut architectural changes, as well as on the modifications of the inflammatory tone and the gut microbiota.


Similar content being viewed by others
References
Kopelman PG (2000) Obesity as a medical problem. Nature 404:635–643
Tordjman J et al (2009) Association between omental adipose tissue macrophages and liver histopathology in morbid obesity: influence of glycemic status. J Hepatol 51:354–362
Skurk T, Alberti-Huber C, Herder C, Hauner H (2007) Relationship between adipocyte size and adipokine expression and secretion. J Clin Endocrinol Metab 92:1023–1033
Engin AB (2017) Adipocyte-macrophage cross-talk in obesity. Adv Exp Med Biol 960:327–343
Cancello R et al (2006) Increased infiltration of macrophages in omental adipose tissue is associated with marked hepatic lesions in morbid human obesity. Diabetes 55:1554–1561
Harman-Boehm I et al (2007) Macrophage infiltration into omental versus subcutaneous fat across different populations: effect of regional adiposity and the comorbidities of obesity. J Clin Endocrinol Metab 92:2240–2247
Dalmas E, Clément K, Guerre-Millo M (2011) Defining macrophage phenotype and function in adipose tissue. Trends Immunol 32:307–314
Aron-Wisnewsky J et al (2009) Human adipose tissue macrophages: m1 and m2 cell surface markers in subcutaneous and omental depots and after weight loss. J Clin Endocrinol Metab 94:4619–4623
Lumeng CN, Bodzin JL, Saltiel AR (2007) Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest 117:175–184
Huber J et al (2008) CC chemokine and CC chemokine receptor profiles in visceral and subcutaneous adipose tissue are altered in human obesity. J Clin Endocrinol Metab 93:3215–3221
Wentworth JM et al (2010) Pro-inflammatory CD11c+CD206+ adipose tissue macrophages are associated with insulin resistance in human obesity. Diabetes 59:1648–1656
Pasarica M et al (2009) Lower total adipocyte number but no evidence for small adipocyte depletion in patients with type 2 diabetes. Diabetes Care 32:900–902
Després J-P, Lemieux I (2006) Abdominal obesity and metabolic syndrome. Nature 444:881–887
Stienstra R et al (2011) Inflammasome is a central player in the induction of obesity and insulin resistance. Proc Natl Acad Sci U S A 108:15324–15329
Dalmas E et al (2014) T cell-derived IL-22 amplifies IL-1β-driven inflammation in human adipose tissue: relevance to obesity and type 2 diabetes. Diabetes 63:1966–1977
Divoux A et al (2012) Mast cells in human adipose tissue: link with morbid obesity, inflammatory status, and diabetes. J Clin Endocrinol Metab 97:E1677–E1685
Rouault C et al (2013) Roles of chemokine ligand-2 (CXCL2) and neutrophils in influencing endothelial cell function and inflammation of human adipose tissue. Endocrinology 154:1069–1079
Viardot A et al (2012) Obesity is associated with activated and insulin resistant immune cells. Diabetes Metab Res Rev 28:447–454
Nishimura S et al (2009) CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med:914–915, 920
Sell H, Habich C, Eckel J (2012) Adaptive immunity in obesity and insulin resistance. Nat Rev Endocrinol 8:709–716
Bäckhed F et al (2004) The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci U S A 101:15718–15723
Ridaura VK et al (2013) Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science 341:1241214
Bohan R et al (2018) Gut microbiota: a potential manipulator for host adipose tissue and energy metabolism. J Nutr Biochem 64:206–217
Cotillard A et al (2013) Dietary intervention impact on gut microbial gene richness. Nature 500:585–588
Le Chatelier E et al (2013) Richness of human gut microbiome correlates with metabolic markers. Nature 500:541–546
Aron-Wisnewsky J et al (2018) Major microbiota dysbiosis in severe obesity: fate after bariatric surgery. Gut. https://doi.org/10.1136/gutjnl-2018-316103
Cani PD et al (2007) Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 56:1761–1772
Cani PD et al (2009) Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut 58:1091–1103
Cani PD et al (2008) Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 57:1470–1481
Genser L et al (2018) Increased jejunal permeability in human obesity is revealed by a lipid challenge and is linked to inflammation and type 2 diabetes. J Pathol. https://doi.org/10.1002/path.5134
Dyson PA (2010) The therapeutics of lifestyle management on obesity. Diabetes Obes Metab 12:941–946
Wing RR, Phelan S (2005) Long-term weight loss maintenance. Am J Clin Nutr 82:222S–225S
Sturm R, Hattori A (2013) Morbid obesity rates continue to rise rapidly in the United States. Int J Obes. 2005 37:889–891
Angrisani L et al (2017) Bariatric surgery and endoluminal procedures: IFSO Worldwide Survey 2014. Obes Surg 27:2279–2289
Fried M et al (2014) Interdisciplinary European guidelines on metabolic and bariatric surgery. Obes Surg 24:42–55
Rubino F et al (2016) Metabolic surgery in the treatment algorithm for type 2 diabetes: a joint statement by international diabetes organizations. Diabetes Care 39:861–877
Dixon JB, Straznicky NE, Lambert EA, Schlaich MP, Lambert GW (2011) Surgical approaches to the treatment of obesity. Nat Rev Gastroenterol Hepatol 8:429–437
Zellmer JD, Mathiason MA, Kallies KJ, Kothari SN (2014) Is laparoscopic sleeve gastrectomy a lower risk bariatric procedure compared with laparoscopic Roux-en-Y gastric bypass? A meta-analysis. Am J Surg 208:903–910; discussion 909–910
Osland E, Yunus RM, Khan S, Memon B, Memon MA (2017) Weight loss outcomes in laparoscopic vertical sleeve gastrectomy (LVSG) versus laparoscopic Roux-en-Y gastric bypass (LRYGB) procedures: a meta-analysis and systematic review of randomized controlled trials. Surg Laparosc Endosc Percutan Tech 27:8–18
Sjöström L et al (2012) Bariatric surgery and long-term cardiovascular events. JAMA J Am Med Assoc 307:56–65
Adams TD et al (2017) Weight and metabolic outcomes 12 years after gastric bypass. N Engl J Med 377:1143–1155
Courcoulas AP et al (2017) Seven-year weight trajectories and health outcomes in the Longitudinal Assessment of Bariatric Surgery (LABS) Study. JAMA Surg. https://doi.org/10.1001/jamasurg.2017.5025
Adams TD et al (2007) Long-term mortality after gastric bypass surgery. N Engl J Med 357:753–761
Schauer PR, Mingrone G, Ikramuddin S, Wolfe B (2016) Clinical outcomes of metabolic surgery: efficacy of glycemic control, weight loss, and remission of diabetes. Diabetes Care 39:902–911
Buse JB et al (2009) How do we define cure of diabetes? Diabetes Care 32:2133–2135
Pournaras DJ et al (2012) Effect of the definition of type II diabetes remission in the evaluation of bariatric surgery for metabolic disorders. Br J Surg 99:100–103
Schauer PR et al (2017) Bariatric surgery versus intensive medical therapy for diabetes—5-year outcomes. N Engl J Med 376:641–651
Debédat J et al (2018) Long-term relapse of type 2 diabetes after Roux-en-Y gastric bypass: prediction and clinical relevance. Diabetes Care. https://doi.org/10.2337/dc18-0567
Mingrone G et al (2012) Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med 366:1577–1585
Thereaux J et al (2017) Long-term follow-up after bariatric surgery in a national cohort. Br J Surg 104:1362–1371
Mingrone G et al (2015) Bariatric–metabolic surgery versus conventional medical treatment in obese patients with type 2 diabetes: 5 year follow-up of an open-label, single-centre, randomised controlled trial. Lancet 386:964–973
Courcoulas AP et al (2015) Three-year outcomes of bariatric surgery vs lifestyle intervention for type 2 diabetes mellitus treatment: a randomized clinical trial. JAMA Surg 150:931–940
Brethauer SA et al (2013) Can diabetes be surgically cured? Long-term metabolic effects of bariatric surgery in obese patients with type 2 diabetes mellitus. Ann Surg 258:628–636; discussion 636–637
Aminian A, Brethauer SA, Kashyap SR, Kirwan JP, Schauer PR (2014) DiaRem score: external validation. Lancet Diabetes Endocrinol 2:12–13
Mehaffey JH et al (2017) Type 2 diabetes remission following gastric bypass: does diarem stand the test of time? Surg Endosc 31:538–542
Still CD et al (2014) Preoperative prediction of type 2 diabetes remission after Roux-en-Y gastric bypass surgery: a retrospective cohort study. Lancet Diabetes Endocrinol 2:38–45
Lee W-J et al (2013) Predicting success of metabolic surgery: age, body mass index, C-peptide, and duration score. Surg Obes Relat Dis Off J Am Soc Bariatr Surg 9:379–384
Aron-Wisnewsky J et al (2017) The advanced-DiaRem score improves prediction of diabetes remission 1 year post-Roux-en-Y gastric bypass. Diabetologia. https://doi.org/10.1007/s00125-017-4371-7
Pucci A et al (2018) Type 2 diabetes remission 2 years post Roux-en-Y gastric bypass and sleeve gastrectomy: the role of the weight loss and comparison of DiaRem and DiaBetter scores. Diabet Med J Br Diabet Assoc 35:360–367
Cotillard A et al (2015) Type 2 diabetes remission after gastric bypass: what is the best prediction tool for clinicians? Obes Surg 25:1128–1132
Lee MH et al (2015) Predictors of long-term diabetes remission after metabolic surgery. J Gastrointest Surg Off J Soc Surg Aliment Tract 19:1015–1021
Wood GC, Mirshahi T, Still CD, Hirsch AG (2016) Association of DiaRem score with cure of type 2 diabetes following bariatric surgery. JAMA Surg 151:779–781
Dicker D, Yahalom R, Comaneshter DS, Vinker S (2016) Long-term outcomes of three types of bariatric surgery on obesity and type 2 diabetes control and remission. Obes Surg 26:1814–1820
Ashrafian H et al (2011) Diabetes resolution and hyperinsulinaemia after metabolic Roux-en-Y gastric bypass. Obes Rev Off J Int Assoc Study Obes 12:e257–e272
Pories WJ et al (1995) Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann Surg 222:339–350; discussion 350–352
Laurenius A et al (2012) Changes in eating behaviour and meal pattern following Roux-en-Y gastric bypass. Int J Obes. 2005 36:348–355
Verger EO et al (2015) Micronutrient and protein deficiencies after gastric bypass and sleeve gastrectomy: a 1-year follow-up. Obes Surg. https://doi.org/10.1007/s11695-015-1803-7
Aron-Wisnewsky J et al (2016) Nutritional and protein deficiencies in the short term following both gastric bypass and gastric banding. PLoS One 11:e0149588
Gras-Miralles B et al (2014) Caloric intake capacity as measured by a standard nutrient drink test helps to predict weight loss after bariatric surgery. Obes Surg 24:2138–2144
le Roux CW et al (2006) Gut hormone profiles following bariatric surgery favor an anorectic state, facilitate weight loss, and improve metabolic parameters. Ann Surg 243:108–114
Lips MA et al (2014) Calorie restriction is a major determinant of the short-term metabolic effects of gastric bypass surgery in obese type 2 diabetic patients. Clin Endocrinol 80:834–842
Steinert RE et al (2017) Ghrelin, CCK, GLP-1, and PYY(3-36): secretory controls and physiological roles in eating and glycemia in health, obesity, and after RYGB. Physiol Rev 97:411–463
Yousseif A et al (2014) Differential effects of laparoscopic sleeve gastrectomy and laparoscopic gastric bypass on appetite, circulating acyl-ghrelin, peptide YY3-36 and active GLP-1 levels in non-diabetic humans. Obes Surg 24:241–252
Laferrère B et al (2008) Effect of weight loss by gastric bypass surgery versus hypocaloric diet on glucose and incretin levels in patients with type 2 diabetes. J Clin Endocrinol Metab 93:2479–2485
Laferrère B et al (2010) Rise of oxyntomodulin in response to oral glucose after gastric bypass surgery in patients with type 2 diabetes. J Clin Endocrinol Metab 95:4072–4076
Cummings DE et al (2002) Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med 346:1623–1630
Holter MM et al (2017) Glucose metabolism after gastric banding and gastric bypass in individuals with type 2 diabetes: weight loss effect. Diabetes Care 40:7–15
Gastaldelli A et al (2016) Short-term effects of laparoscopic adjustable gastric banding versus Roux-en-Y gastric bypass. Diabetes Care. https://doi.org/10.2337/dc15-2823
Dimitriadis GK, Randeva MS, Miras AD (2017) Potential hormone mechanisms of bariatric surgery. Curr Obes Rep 6:253–265
Laferrère B, Pattou F (2018) Weight-independent mechanisms of glucose control after Roux-en-Y gastric bypass. Front Endocrinol 9
Castagneto Gissey L, Casella Mariolo J, Mingrone G (2018) Intestinal peptide changes after bariatric and minimally invasive surgery: relation to diabetes remission. Peptides 100:114–122
Wang G et al (2012) Accelerated gastric emptying but no carbohydrate malabsorption 1 year after gastric bypass surgery (GBP). Obes Surg 22:1263–1267
Stano S et al (2017) Effect of meal size and texture on gastric pouch emptying and glucagon-like peptide 1 after gastric bypass surgery. Surg Obes Relat Dis Off J Am Soc Bariatr Surg 13:1975–1983
Jacobsen SH et al (2013) Effects of gastric bypass surgery on glucose absorption and metabolism during a mixed meal in glucose-tolerant individuals. Diabetologia 56:2250–2254
Vilsbøll T, Krarup T, Deacon CF, Madsbad S, Holst JJ (2001) Reduced postprandial concentrations of intact biologically active glucagon-like peptide 1 in type 2 diabetic patients. Diabetes 50:609–613
Knop FK et al (2007) Reduced incretin effect in type 2 diabetes: cause or consequence of the diabetic state? Diabetes 56:1951–1959
Xu G, Stoffers DA, Habener JF, Bonner-Weir S (1999) Exendin-4 stimulates both beta-cell replication and neogenesis, resulting in increased beta-cell mass and improved glucose tolerance in diabetic rats. Diabetes 48:2270–2276
Vetter ML et al (2015) GLP-1 plays a limited role in improved glycemia shortly after Roux-en-Y gastric bypass: a comparison with intensive lifestyle modification. Diabetes 64:434–446
Shah M et al (2014) Contribution of endogenous glucagon-like peptide 1 to glucose metabolism after Roux-en-Y gastric bypass. Diabetes 63:483–493
Mokadem M, Zechner JF, Margolskee RF, Drucker DJ, Aguirre V (2014) Effects of Roux-en-Y gastric bypass on energy and glucose homeostasis are preserved in two mouse models of functional glucagon-like peptide-1 deficiency. Mol Metab 3:191–201
Wilson-Pérez HE et al (2013) Vertical sleeve gastrectomy is effective in two genetic mouse models of glucagon-like Peptide 1 receptor deficiency. Diabetes 62:2380–2385
Garibay D et al (2016) β-Cell glucagon-like peptide-1 receptor contributes to improved glucose tolerance after vertical sleeve gastrectomy. Endocrinology 157:3405–3409
Chambers AP et al (2017) The role of pancreatic preproglucagon in glucose homeostasis in mice. Cell Metab 25:927–934.e3
Marchetti P et al (2012) A local glucagon-like peptide 1 (GLP-1) system in human pancreatic islets. Diabetologia 55:3262–3272
Garibay D et al (2018) β Cell GLP-1R signaling alters α cell proglucagon processing after vertical sleeve gastrectomy in mice. Cell Rep 23:967–973
Service GJ et al (2005) Hyperinsulinemic hypoglycemia with nesidioblastosis after gastric-bypass surgery. N Engl J Med 353:249–254
Dadheech N, Garrel D, Buteau J (2018) Evidence of unrestrained beta-cell proliferation and neogenesis in a patient with hyperinsulinemic hypoglycemia after gastric bypass surgery. Islets 10:213–220
Meier JJ, Butler AE, Galasso R, Butler PC (2006) Hyperinsulinemic hypoglycemia after gastric bypass surgery is not accompanied by islet hyperplasia or increased-cell turnover. Diabetes Care 29:1554–1559
Lautenbach A et al (2018) Adaptive changes in pancreas post Roux-en-Y gastric bypass induced weight loss. Diabetes Metab Res Rev 34:e3025
Gaborit B et al (2015) Ectopic fat storage in the pancreas using 1H-MRS: importance of diabetic status and modulation with bariatric surgery-induced weight loss. Int J Obes 2005(39):480–487
Zhou X, Qian B, Ji N, Lui C, Liu Z, Li B, Zhou H, Yan C (2016) Pancreatic hyperplasia after gastric bypass surgery in a GK rat model of non-obese type 2 diabetes. J Endocrinol 228:13–23
Zhang S, Guo W, Wu J, Gong L, Li Q, Xiao X, Zhang J, Wang Z (2017) Increased β-cell mass in obese rats after gastric bypass: a potential mechanism for improving glycemic control. Med Sci Monit 23:2151–2158
McGavigan AK, Garibay D, Henseler ZM, Chen J, Bettaieb A, Haj FG, Ley RE, Chouinard ML, Cummings BP (2017) TGR5 contributes to glucoregulatory improvements after vertical sleeve gastrectomy in mice. Gut 66:226–234
Cummings BP, Bettaieb A, Graham JL, Kim J, Ma F, Shibata N, Stanhope KL, Giulivi C, Hansen F, Jelsing J, Vrang N, Kowala M, Chouinard ML, Haj FG, Havel PJ (2013) Bile-acid-mediated decrease in endoplasmic reticulum stress: a potential contributor to the metabolic benefits of ileal interposition surgery in UCD-T2DM rats. Dis Model Mech 6:443–456
Dutia R, Brakoniecki K, Bunker P, Paultre F, Homel P, Carpentier AC, McGinty J, Laferrere B (2014) Limited recovery of β-cell function after gastric bypass despite clinical diabetes remission. Diabetes 63:1214–1223
Drucker DJ, Shi Q, Crivici A, Sumner-Smith M, Tavares W, Hill M, DeForest L, Cooper S, Brubaker PL (1997) Regulation of the biological activity of glucagon-like peptide 2 in vivo by dipeptidyl peptidase IV. Nat Biotechnol 15:673–677
Drucker DJ, Erlich P, Asa SL, Brubaker PL (1996) Induction of intestinal epithelial proliferation by glucagon-like peptide 2. Proc Natl Acad Sci U S A 93:7911–7916
le Roux CW, Borg C, Wallis K, Vincent RP, Bueter M, Goodlad R, Ghatei MA, Patel A, Bloom SR, Aylwin SJB (2010) Gut hypertrophy after gastric bypass is associated with increased glucagon-like peptide 2 and intestinal crypt cell proliferation. Ann Surg 252:50–56
Cazzo E, Pareja JC, Chaim EA, Geloneze B, Barreto MRL, Magro DO (2017) GLP-1 and GLP-2 levels are correlated with satiety regulation after Roux-en-Y gastric bypass: results of an exploratory prospective study. Obes Surg 27:703–708
Jeppesen PB, Hartmann B, Thulesen J, Graff J, Lohmann J, Hansen BS, Tofteng F, Poulsen SS, Madsen JL, Holst JJ, Mortensen PB (2001) Glucagon-like peptide 2 improves nutrient absorption and nutritional status in short-bowel patients with no colon. Gastroenterology 120:806–815
Iqbal CW, Qandeel HG, Zheng Y, Duenes JA, Sarr MG (2008) Mechanisms of ileal adaptation for glucose absorption after proximal-based small bowel resection. J Gastrointest Surg Off J Soc Surg Aliment Tract 12:1854–1864; discussion 1864–1865
Li B, Lu Y, Srikant CB, Gao Z-H, Liu J-L (2013) Intestinal adaptation and Reg gene expression induced by antidiabetic duodenal-jejunal bypass surgery in Zucker fatty rats. Am J Physiol Gastrointest Liver Physiol 304:G635–G645
Taqi E, Wallace LE, de Heuvel E, Chelikani PK, Zheng H, Berthoud HR, Holst JJ, Sigalet DL (2010) The influence of nutrients, biliary-pancreatic secretions, and systemic trophic hormones on intestinal adaptation in a Roux-en-Y bypass model. J Pediatr Surg 45:987–995
Cavin J-B, Voitellier E, Cluzeaud F, Kapel N, Marmuse JP, Chevallier JM, Msika S, Bado A, le Gall M (2016) Malabsorption and intestinal adaptation after one anastomosis gastric bypass compared with Roux-en-Y gastric bypass in rats. Am J Physiol-Gastrointest Liver Physiol 311:G492–G500
Cavin J-B et al (2016) Differences in alimentary glucose absorption and intestinal disposal of blood glucose after Roux-en-Y gastric bypass vs sleeve gastrectomy. Gastroenterology 150:454–464.e9
Troy S, Soty M, Ribeiro L, Laval L, Migrenne S, Fioramonti X, Pillot B, Fauveau V, Aubert R, Viollet B, Foretz M, Leclerc J, Duchampt A, Zitoun C, Thorens B, Magnan C, Mithieux G, Andreelli F (2008) Intestinal gluconeogenesis is a key factor for early metabolic changes after gastric bypass but not after gastric lap-band in mice. Cell Metab 8:201–211
Saeidi N, Meoli L, Nestoridi E, Gupta NK, Kvas S, Kucharczyk J, Bonab AA, Fischman AJ, Yarmush ML, Stylopoulos N (2013) Reprogramming of intestinal glucose metabolism and glycemic control in rats after gastric bypass. Science 341:406–410
Baud G, Daoudi M, Hubert T, Raverdy V, Pigeyre M, Hervieux E, Devienne M, Ghunaim M, Bonner C, Quenon A, Pigny P, Klein A, Kerr-Conte J, Gmyr V, Caiazzo R, Pattou F (2016) Bile diversion in Roux-en-Y gastric bypass modulates sodium-dependent glucose intestinal uptake. Cell Metab 23:547–553
Thomas C, Gioiello A, Noriega L, Strehle A, Oury J, Rizzo G, Macchiarulo A, Yamamoto H, Mataki C, Pruzanski M, Pellicciari R, Auwerx J, Schoonjans K (2009) TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab 10:167–177
Kohli R, Bradley D, Setchell KD, Eagon JC, Abumrad N, Klein S (2013) Weight loss induced by Roux-en-Y gastric bypass but not laparoscopic adjustable gastric banding increases circulating bile acids. J Clin Endocrinol Metab 98:E708–E712
Patti M-E, Houten SM, Bianco AC, Bernier R, Larsen PR, Holst JJ, Badman MK, Maratos-Flier E, Mun EC, Pihlajamaki J, Auwerx J, Goldfine AB (2009) Serum bile acids are higher in humans with prior gastric bypass: potential contribution to improved glucose and lipid metabolism. Obes Silver Spring Md 17:1671–1677
Ryan KK, Tremaroli V, Clemmensen C, Kovatcheva-Datchary P, Myronovych A, Karns R, Wilson-Pérez HE, Sandoval DA, Kohli R, Bäckhed F, Seeley RJ (2014) FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature 509:183–188
Albaugh VL, Flynn CR, Cai S, Xiao Y, Tamboli RA, Abumrad NN (2015) Early increases in bile acids post Roux-en-Y gastric bypass are driven by insulin-sensitizing, secondary bile acids. J Clin Endocrinol Metab 100:E1225–E1233
Duboc H et al (2018) Roux-en-Y gastric-bypass and sleeve gastrectomy induces specific shifts of the gut microbiota without altering the metabolism of bile acids in the intestinal lumen. Int J Obes 1. https://doi.org/10.1038/s41366-018-0015-3
Wu T, Bound MJ, Standfield SD, Jones KL, Horowitz M, Rayner CK (2013) Effects of taurocholic acid on glycemic, glucagon-like peptide-1, and insulin responses to small intestinal glucose infusion in healthy humans. J Clin Endocrinol Metab 98:E718–E722
Li F, Jiang C, Krausz KW, Li Y, Albert I, Hao H, Fabre KM, Mitchell JB, Patterson AD, Gonzalez FJ (2013) Microbiome remodelling leads to inhibition of intestinal farnesoid X receptor signalling and decreased obesity. Nat Commun 4:2384
Sun L et al (2018) Gut microbiota and intestinal FXR mediate the clinical benefits of metformin. Nat Med 1. https://doi.org/10.1038/s41591-018-0222-4
Fang S, Suh JM, Reilly SM, Yu E, Osborn O, Lackey D, Yoshihara E, Perino A, Jacinto S, Lukasheva Y, Atkins AR, Khvat A, Schnabl B, Yu RT, Brenner DA, Coulter S, Liddle C, Schoonjans K, Olefsky JM, Saltiel AR, Downes M, Evans RM (2015) Intestinal FXR agonism promotes adipose tissue browning and reduces obesity and insulin resistance. Nat Med 21:159–165
Sayin SI, Wahlström A, Felin J, Jäntti S, Marschall HU, Bamberg K, Angelin B, Hyötyläinen T, Orešič M, Bäckhed F (2013) Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab 17:225–235
Furet J-P, Kong LC, Tap J, Poitou C, Basdevant A, Bouillot JL, Mariat D, Corthier G, Dore J, Henegar C, Rizkalla S, Clement K (2010) Differential adaptation of human gut microbiota to bariatric surgery-induced weight loss. Diabetes 59:3049–3057
Kong L-C, Tap J, Aron-Wisnewsky J, Pelloux V, Basdevant A, Bouillot JL, Zucker JD, Doré J, Clément K (2013) Gut microbiota after gastric bypass in human obesity: increased richness and associations of bacterial genera with adipose tissue genes. Am J Clin Nutr 98:16–24
Guo Y et al. (2017) Modulation of the gut microbiome: a systematic review of the effect of bariatric surgery. Eur J Endocrinol.https://doi.org/10.1530/EJE-17-0403
Graessler J, Qin Y, Zhong H, Zhang J, Licinio J, Wong ML, Xu A, Chavakis T, Bornstein AB, Ehrhart-Bornstein M, Lamounier-Zepter V, Lohmann T, Wolf T, Bornstein SR (2013) Metagenomic sequencing of the human gut microbiome before and after bariatric surgery in obese patients with type 2 diabetes: correlation with inflammatory and metabolic parameters. Pharm J 13:514–522
Tremaroli V, Karlsson F, Werling M, Ståhlman M, Kovatcheva-Datchary P, Olbers T, Fändriks L, le Roux CW, Nielsen J, Bäckhed F (2015) Roux-en-Y gastric bypass and vertical banded gastroplasty induce long-term changes on the human gut microbiome contributing to fat mass regulation. Cell Metab 22:228–238
Zhang H, DiBaise JK, Zuccolo A, Kudrna D, Braidotti M, Yu Y, Parameswaran P, Crowell MD, Wing R, Rittmann BE, Krajmalnik-Brown R (2009) Human gut microbiota in obesity and after gastric bypass. Proc Natl Acad Sci U S A 106:2365–2370
Aron-Wisnewsky J, Doré J, Clement K (2012) The importance of the gut microbiota after bariatric surgery. Nat Rev Gastroenterol Hepatol 9:590–598
Rothschild D, Weissbrod O, Barkan E, Kurilshikov A, Korem T, Zeevi D, Costea PI, Godneva A, Kalka IN, Bar N, Shilo S, Lador D, Vila AV, Zmora N, Pevsner-Fischer M, Israeli D, Kosower N, Malka G, Wolf BC, Avnit-Sagi T, Lotan-Pompan M, Weinberger A, Halpern Z, Carmi S, Fu J, Wijmenga C, Zhernakova A, Elinav E, Segal E (2018) Environment dominates over host genetics in shaping human gut microbiota. Nature 555:210–215
Shin N-R, Lee JC, Lee HY, Kim MS, Whon TW, Lee MS, Bae JW (2014) An increase in the Akkermansia spp. population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice. Gut 63:727–735
Forslund K et al (2015) Disentangling the effects of type 2 diabetes and metformin on the human gut microbiota. Nature 528:262–266
Jackson MA, Verdi S, Maxan ME, Shin CM, Zierer J, Bowyer RCE, Martin T, Williams FMK, Menni C, Bell JT, Spector TD, Steves CJ (2018) Gut microbiota associations with common diseases and prescription medications in a population-based cohort. Nat Commun 9:2655
Vesper B, Jawdi A, Altman K, Haines III G, Tao L, Radosevich J (2009) The effect of proton pump inhibitors on the human microbiota. Curr Drug Metab 10:84–89
Imhann F, Bonder MJ, Vich Vila A, Fu J, Mujagic Z, Vork L, Tigchelaar EF, Jankipersadsing SA, Cenit MC, Harmsen HJM, Dijkstra G, Franke L, Xavier RJ, Jonkers D, Wijmenga C, Weersma RK, Zhernakova A (2016) Proton pump inhibitors affect the gut microbiome. Gut 65:740–748
Ward EK, Schuster DP, Stowers KH, Royse AK, Ir D, Robertson CE, Frank DN, Austin GL (2014) The effect of PPI use on human gut microbiota and weight loss in patients undergoing laparoscopic Roux-en-Y gastric bypass. Obes Surg 24:1567–1571
Plovier H, Everard A, Druart C, Depommier C, van Hul M, Geurts L, Chilloux J, Ottman N, Duparc T, Lichtenstein L, Myridakis A, Delzenne NM, Klievink J, Bhattacharjee A, van der Ark KCH, Aalvink S, Martinez LO, Dumas ME, Maiter D, Loumaye A, Hermans MP, Thissen JP, Belzer C, de Vos WM, Cani PD (2016) A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat Med 23:107–113. https://doi.org/10.1038/nm.4236
Dao MC et al. (2015) Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: relationship with gut microbiome richness and ecology. Gut. https://doi.org/10.1136/gutjnl-2014-308778
de la Cuesta-Zuluaga J, Mueller NT, Corrales-Agudelo V, Velásquez-Mejía EP, Carmona JA, Abad JM, Escobar JS (2017) Metformin is associated with higher relative abundance of mucin-degrading Akkermansia muciniphila and several short-chain fatty acid–producing microbiota in the gut. Diabetes Care 40:54–62
Murphy R et al. (2017) Laparoscopic sleeve gastrectomy versus banded Roux-en-Y gastric bypass for diabetes and obesity: a prospective randomised double-blind trial. Obes Surg. 1–10. https://doi.org/10.1007/s11695-017-2872-6
Liou AP et al (2013) Conserved shifts in the gut microbiota due to gastric bypass reduce host weight and adiposity. Sci Transl Med 5:178ra41
Arora T, Seyfried F, Docherty NG, Tremaroli V, le Roux CW, Perkins R, Bäckhed F (2017) Diabetes-associated microbiota in fa/fa rats is modified by Roux-en-Y gastric bypass. ISME J 11:2035–2046
Casselbrant A, Elias E, Fändriks L, Wallenius V (2015) Expression of tight-junction proteins in human proximal small intestinal mucosa before and after Roux-en-Y gastric bypass surgery. Surg Obes Relat Dis 11:45–53
Monte SV, Caruana JA, Ghanim H, Sia CL, Korzeniewski K, Schentag JJ, Dandona P (2012) Reduction in endotoxemia, oxidative and inflammatory stress, and insulin resistance after Roux-en-Y gastric bypass surgery in patients with morbid obesity and type 2 diabetes mellitus. Surgery 151:587–593
Yang P-J, Lee WJ, Tseng PH, Lee PH, Lin MT, Yang WS (2014) Bariatric surgery decreased the serum level of an endotoxin-associated marker: lipopolysaccharide-binding protein. Surg Obes Relat Dis 10:1182–1187
Trøseid M et al (2013) Plasma lipopolysaccharide is closely associated with glycemic control and abdominal obesity: evidence from bariatric surgery. Diabetes Care 36:3627–3632
Clemente-Postigo M, Roca-Rodriguez MM, Camargo A, Ocaña-Wilhelmi L, Cardona F, Tinahones FJ (2014) Lipopolysaccharide and lipopolysaccharide-binding protein levels and their relationship to early metabolic improvement after bariatric surgery. Surg Obes Relat Dis Off J Am Soc Bariatr Surg 11:933–939. https://doi.org/10.1016/j.soard.2014.11.030
Guo Y, Liu C-Q, Liu G-P, Huang Z-P, Zou D-J (2019) Roux-en-Y gastric bypass decreases endotoxemia and inflammatory stress in association with improvement of gut permeability in obese diabetic rats. J Diabetes. https://doi.org/10.1111/1753-0407.12906
le Roux CW, Bueter M, Theis N, Werling M, Ashrafian H, Löwenstein C, Athanasiou T, Bloom SR, Spector AC, Olbers T, Lutz TA (2011) Gastric bypass reduces fat intake and preference. Am J Physiol-Regul Integr Comp Physiol 301:R1057–R1066
Kuribayashi H, Miyata M, Yamakawa H, Yoshinari K, Yamazoe Y (2012) Enterobacteria-mediated deconjugation of taurocholic acid enhances ileal farnesoid X receptor signaling. Eur J Pharmacol 697:132–138
Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, Liang S, Zhang W, Guan Y, Shen D, Peng Y, Zhang D, Jie Z, Wu W, Qin Y, Xue W, Li J, Han L, Lu D, Wu P, Dai Y, Sun X, Li Z, Tang A, Zhong S, Li X, Chen W, Xu R, Wang M, Feng Q, Gong M, Yu J, Zhang Y, Zhang M, Hansen T, Sanchez G, Raes J, Falony G, Okuda S, Almeida M, LeChatelier E, Renault P, Pons N, Batto JM, Zhang Z, Chen H, Yang R, Zheng W, Li S, Yang H, Wang J, Ehrlich SD, Nielsen R, Pedersen O, Kristiansen K, Wang J (2012) A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 490:55–60
Torres-Leal FL, Fonseca-Alaniz MH, Teodoro GFR, de Capitani MD, Vianna D, Pantaleão LC, Matos-Neto EM, Rogero MM, Donato J, Tirapegui J (2011) Leucine supplementation improves adiponectin and total cholesterol concentrations despite the lack of changes in adiposity or glucose homeostasis in rats previously exposed to a high-fat diet. Nutr Metab 8:62
Cota D et al (2006) Hypothalamic mTOR signaling regulates food intake. Science 312:927–930
Binder E, Bermúdez-Silva FJ, Elie M, Leste-Lasserre T, Belluomo I, Clark S, Duchampt A, Mithieux G, Cota D (2014) Leucine supplementation modulates fuel substrates utilization and glucose metabolism in previously obese mice. Obes Silver Spring Md 22:713–720
Newgard CB, An J, Bain JR, Muehlbauer MJ, Stevens RD, Lien LF, Haqq AM, Shah SH, Arlotto M, Slentz CA, Rochon J, Gallup D, Ilkayeva O, Wenner BR, Yancy WS Jr, Eisenson H, Musante G, Surwit RS, Millington DS, Butler MD, Svetkey LP (2009) A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab 9:311–326
Pedersen HK, Gudmundsdottir V, Nielsen HB, Hyotylainen T, Nielsen T, Jensen BAH, Forslund K, Hildebrand F, Prifti E, Falony G, le Chatelier E, Levenez F, Doré J, Mattila I, Plichta DR, Pöhö P, Hellgren LI, Arumugam M, Sunagawa S, Vieira-Silva S, Jørgensen T, Holm JB, Trošt K, Consortium MHIT, Kristiansen K, Brix S, Raes J, Wang J, Hansen T, Bork P, Brunak S, Oresic M, Ehrlich SD, Pedersen O (2016) Human gut microbes impact host serum metabolome and insulin sensitivity. Nature 535:376–381
Stancáková A et al (2012) Hyperglycemia and a common variant of GCKR are associated with the levels of eight amino acids in 9,369 Finnish men. Diabetes 61:1895–1902
Cheng S, Rhee EP, Larson MG, Lewis GD, McCabe EL, Shen D, Palma MJ, Roberts LD, Dejam A, Souza AL, Deik AA, Magnusson M, Fox CS, O'Donnell CJ, Vasan RS, Melander O, Clish CB, Gerszten RE, Wang TJ (2012) Metabolite profiling identifies pathways associated with metabolic risk in humans. Circulation 125:2222–2231
Lanza IR, Zhang S, Ward LE, Karakelides H, Raftery D, Nair KS (2010) Quantitative metabolomics by 1H-NMR and LC-MS/MS confirms altered metabolic pathways in diabetes. PLoS One 5:e10538
Wang TJ, Larson MG, Vasan RS, Cheng S, Rhee EP, McCabe E, Lewis GD, Fox CS, Jacques PF, Fernandez C, O'Donnell CJ, Carr SA, Mootha VK, Florez JC, Souza A, Melander O, Clish CB, Gerszten RE (2011) Metabolite profiles and the risk of developing diabetes. Nat Med 17:448–453
Yoon M-S (2016) The emerging role of branched-chain amino acids in insulin resistance and metabolism. Nutrients 8
Melnik BC (2012) Leucine signaling in the pathogenesis of type 2 diabetes and obesity. World J Diabetes 3:38–53
Morales NB & Plata CA. de. (2012) Role of AKT/mTORC1 pathway in pancreatic β-cell proliferation. Colomb Médica 43, 235–243–243
Wei X, Yan X, Zou D, Yang Z, Wang X, Liu W, Wang S, Li X, Han J, Huang L, Yuan J (2013) Abnormal fecal microbiota community and functions in patients with hepatitis B liver cirrhosis as revealed by a metagenomic approach. BMC Gastroenterol 13:175
Laferrère B et al (2011) Differential metabolic impact of gastric bypass surgery versus dietary intervention in obese diabetic subjects despite identical weight loss. Sci Transl Med 3:80re2–80re2
Gralka E, Luchinat C, Tenori L, Ernst B, Thurnheer M, Schultes B (2015) Metabolomic fingerprint of severe obesity is dynamically affected by bariatric surgery in a procedure-dependent manner. Am J Clin Nutr 102:1313–1322
Lips MA, van Klinken JB, van Harmelen V, Dharuri HK, ’t Hoen PAC, Laros JFJ, van Ommen GJ, Janssen IM, van Ramshorst B, van Wagensveld BA, Swank DJ, van Dielen F, Dane A, Harms A, Vreeken R, Hankemeier T, Smit JWA, Pijl H, Willems van Dijk K (2014) Roux-en-Y gastric bypass surgery, but not calorie restriction, reduces plasma branched-chain amino acids in obese women independent of weight loss or the presence of type 2 diabetes. Diabetes Care 37:3150–3156
Lopes TIB, Geloneze B, Pareja JC, Calixto AR, Ferreira MMC, Marsaioli AJ (2016) “Omics” prospective monitoring of bariatric surgery: Roux-En-Y gastric bypass outcomes using mixed-meal tolerance test and time-resolved 1H NMR-based metabolomics. OMICS J Integr Biol 20:415–423
Magkos F, Bradley D, Schweitzer GG, Finck BN, Eagon JC, Ilkayeva O, Newgard CB, Klein S (2013) Effect of Roux-en-Y gastric bypass and laparoscopic adjustable gastric banding on branched-chain amino acid metabolism. Diabetes 62:2757–2761
Narath SH, Mautner SI, Svehlikova E, Schultes B, Pieber TR, Sinner FM, Gander E, Libiseller G, Schimek MG, Sourij H, Magnes C (2016) An untargeted metabolomics approach to characterize short-term and long-term metabolic changes after bariatric surgery. PLoS One 11:e0161425
Arora T, Velagapudi V, Pournaras DJ, Welbourn R, le Roux CW, Orešič M, Bäckhed F (2015) Roux-en-Y gastric bypass surgery induces early plasma metabolomic and lipidomic alterations in humans associated with diabetes remission. PLoS One 10:e0126401
De Haes W et al (2014) Metformin promotes lifespan through mitohormesis via the peroxiredoxin PRDX-2. Proc Natl Acad Sci 111:E2501–E2509
Walford GA, Davis J, Warner AS, Ackerman RJ, Billings LK, Chamarthi B, Fanelli RR, Hernandez AM, Huang C, Khan SQ, Littleton KR, Lo J, McCarthy RM, Rhee EP, Deik A, Stolerman E, Taylor A, Hudson MS, Wang TJ, Altshuler D, Grant RW, Clish CB, Gerszten RE, Florez JC (2013) Branched chain and aromatic amino acids change acutely following two medical therapies for type 2 diabetes mellitus. Metabolism 62:1772–1778
Cotillard A, Poitou C, Torcivia A, Bouillot JL, Dietrich A, Klöting N, Grégoire C, Lolmede K, Blüher M, Clément K (2014) Adipocyte size threshold matters: link with risk of type 2 diabetes and improved insulin resistance after gastric bypass. J Clin Endocrinol Metab 99:E1466–E1470
Dalmas E, Rouault C, Abdennour M, Rovere C, Rizkalla S, Bar-Hen A, Nahon JL, Bouillot JL, Guerre-Millo M, Clément K, Poitou C (2011) Variations in circulating inflammatory factors are related to changes in calorie and carbohydrate intakes early in the course of surgery-induced weight reduction. Am J Clin Nutr 94:450–458
Cancello R, Henegar C, Viguerie N, Taleb S, Poitou C, Rouault C, Coupaye M, Pelloux V, Hugol D, Bouillot JL, Bouloumie A, Barbatelli G, Cinti S, Svensson PA, Barsh GS, Zucker JD, Basdevant A, Langin D, Clement K (2005) Reduction of macrophage infiltration and chemoattractant gene expression changes in white adipose tissue of morbidly obese subjects after surgery-induced weight loss. Diabetes 54:2277–2286
Liu Y, Aron-Wisnewsky J, Marcelin G, Genser L, le Naour G, Torcivia A, Bauvois B, Bouchet S, Pelloux V, Sasso M, Miette V, Tordjman J, Clément K (2016) Accumulation and changes in composition of collagens in subcutaneous adipose tissue after bariatric surgery. J Clin Endocrinol Metab 101:293–304
García-Rubio J, León J, Redruello-Romero A, Pavón E, Cozar A, Tamayo F, Caba-Molina M, Salmerón J, Carazo Á (2018) Cytometric analysis of adipose tissue reveals increments of adipocyte progenitor cells after weight loss induced by bariatric surgery. Sci Rep 8:15203
Faria G, Pestana D, Aral M, Preto J, Norberto S, Calhau C, Guimarães JT, Taveira-Gomes A (2014) Metabolic score: insights on the development and prediction of remission of metabolic syndrome after gastric bypass. Ann Surg 260:279–286
Zamarron BF et al. (2016) Macrophage proliferation sustains adipose tissue inflammation in formerly obese mice. Diabetes. https://doi.org/10.2337/db16-0500
Poitou C, Dalmas E, Renovato M, Benhamo V, Hajduch F, Abdennour M, Kahn JF, Veyrie N, Rizkalla S, Fridman WH, Sautès-Fridman C, Clément K, Cremer I (2011) CD14dimCD16+ and CD14+CD16+ monocytes in obesity and during weight loss: relationships with fat mass and subclinical atherosclerosis. Arterioscler Thromb Vasc Biol 31:2322–2330
Rogacev KS, Ulrich C, Blomer L, Hornof F, Oster K, Ziegelin M, Cremers B, Grenner Y, Geisel J, Schlitt A, Kohler H, Fliser D, Girndt M, Heine GH (2010) Monocyte heterogeneity in obesity and subclinical atherosclerosis. Eur Heart J 31:369–376
Pecht T, Haim Y, Bashan N, Shapiro H, Harman-Boehm I, Kirshtein B, Clément K, Shai I, Rudich A (2016) Circulating blood monocyte subclasses and lipid-laden adipose tissue macrophages in human obesity. PLoS One 11:e0159350
Chen H et al. Change in gut microbiota is correlated with alterations in the surface molecule expression of monocytes after Roux-en-Y gastric bypass surgery in obese type 2 diabetic patients. 12
Viardot A, Lord RV, Samaras K (2010) The effects of weight loss and gastric banding on the innate and adaptive immune system in type 2 diabetes and prediabetes. J Clin Endocrinol Metab 95:2845–2850
Samaras K, Viardot A, Botelho NK, Jenkins A, Lord RV (2013) Immune cell-mediated inflammation and the early improvements in glucose metabolism after gastric banding surgery. Diabetologia 56:2564–2572
Magalhaes I, Pingris K, Poitou C, Bessoles S, Venteclef N, Kiaf B, Beaudoin L, da Silva J, Allatif O, Rossjohn J, Kjer-Nielsen L, McCluskey J, Ledoux S, Genser L, Torcivia A, Soudais C, Lantz O, Boitard C, Aron-Wisnewsky J, Larger E, Clément K, Lehuen A (2015) Mucosal-associated invariant T cell alterations in obese and type 2 diabetic patients. J Clin Invest 125:1752–1762
Touch S et al. (2018) Mucosal-associated invariant T (MAIT) cells are depleted and prone to apoptosis in cardiometabolic disorders. FASEB J Off Publ Fed Am Soc Exp Biol. https://doi.org/10.1096/fj.201800052RR
Tastan C, Karhan E, Zhou W, Fleming E, Voigt AY, Yao X, Wang L, Horne M, Placek L, Kozhaya L, Oh J, Unutmaz D (2018) Tuning of human MAIT cell activation by commensal bacteria species and MR1-dependent T-cell presentation. Mucosal Immunol 11:1591–1605
Acknowledgments
The authors would like to thank Dr Tim Swartz for the careful English-language review of the manuscript, as well as Les Laboratoires Servier for the use of icons provided through the SMART (Servier Medical ART) image collection (https://smart.servier.com/). JAW received grant from Institut Benjamin Delessert and Société Francophone du Diabète (SFD) and KC received an award from the Fondation de France.
Funding
Funding to support NutriOmics research Unit activity on this reviewed topic were obtained from European Union’s Seventh Framework Program (FP7) for research, technological development and demonstration under grant agreement HEALTH-F4-2012-305312 (Metacardis) and from the French national program “Investissement d’Avenir” FORCE, the Metagenopolis grant ANR-11-DPBS-0001 and the ANR-10-IAHU-05, (Institute of Cardiometabolism and Nutrition), from the National Agency of Research ANR-OB MAIT, CAPTOR, and from INSERM (to CA as program “Accueil Jeune Chercheur”).
Author information
Authors and Affiliations
Contributions
JD and CA contributed to the research, discussion of content, and writing of this manuscript; J.A.W contributed to the research, discussion of content, writing, and editing of this manuscript; and K.C. contributed to the discussion of content, writing, and reviewing/editing of the manuscript before submission. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
This article is a contribution to the special issue on Inflammation and Type 2 Diabetes - Guest Editor: Marc Y. Donath
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Judith Aron-Wisnewsky and Karine Clément share last-authorship.
Rights and permissions
About this article
Cite this article
Debédat, J., Amouyal, C., Aron-Wisnewsky, J. et al. Impact of bariatric surgery on type 2 diabetes: contribution of inflammation and gut microbiome?. Semin Immunopathol 41, 461–475 (2019). https://doi.org/10.1007/s00281-019-00738-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00281-019-00738-3