Abstract
Purpose
Model-based methods can predict pediatric exposure and support initial dose selection. The aim of this study was to evaluate the performance of allometric scaling of population pharmacokinetic (popPK) versus physiologically based pharmacokinetic (PBPK) models in predicting the exposure of tyrosine kinase inhibitors (TKIs) for pediatric patients (≥ 2 years), based on adult data. The drugs imatinib, sunitinib and pazopanib were selected as case studies due to their complex PK profiles including high inter-patient variability, active metabolites, time-varying clearances and non-linear absorption.
Methods
Pediatric concentration measurements and adult popPK models were derived from the literature. Adult PBPK models were generated in PK-Sim® using available physicochemical properties, calibrated to adult data when needed. PBPK and popPK models for the pediatric populations were translated from the models for adults and were used to simulate concentration-time profiles that were compared to the observed values.
Results
Ten pediatric datasets were collected from the literature. While both types of models captured the concentration-time profiles of imatinib, its active metabolite, sunitinib and pazopanib, the PBPK models underestimated sunitinib metabolite concentrations. In contrast, allometrically scaled popPK simulations accurately predicted all concentration-time profiles. Trough concentration (Ctrough) predictions from the popPK model fell within a 2-fold range for all compounds, while 3 out of 5 PBPK predictions exceeded this range for the imatinib and sunitinib metabolite concentrations.
Conclusion
Based on the identified case studies it appears that allometric scaling of popPK models is better suited to predict exposure of TKIs in pediatric patients ≥ 2 years. This advantage may be attributed to the stable enzyme expression patterns from 2 years old onwards, which can be easily related to adult levels through allometric scaling. In some instances, both methods performed comparably. Understanding where discrepancies between the model methods arise, can further inform model development and ultimately support pediatric dose selection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Malignancies are one of the major causes of morbidities and mortalities among children in the developed world [1]. Tyrosine kinase inhibitors (TKIs) target specific proteins, receptors and enzymes involved in molecular pathways that are crucial for cancer growth and proliferation and are thus considered safer than traditional chemotherapies [2]. Despite the fact that TKIs are part of the treatment regimen for various solid and hematological malignancies, only a few are approved for pediatric indications [2, 3].
Selection of an adequate dose represents an important step in expanding regulatory approval of TKIs towards the pediatric population. The pediatric regulation by the European Union and the amendment of the Food and Drug Administration’s (FDA) Pediatric Research Equity and Best Pharmaceuticals for Children Act (2006–2007) have instigated more focus on dose optimization in children. During dose selection, differences in both the pharmacokinetics (PK) and pharmacodynamics (PD) of a drug can be taken into account [4]. However, given the redundancy in the mechanism of action of TKIs, differences between children and adults are most likely to originate from discrepancies in drug PK and, as such, exposure matching is desired [5,6,7,8]. Indeed, the majority of Phase I investigations in the pediatric population, undertaken for dose selection for subsequent evaluation in Phase II trials concerning safety and efficacy evaluations, have focused on exposure matching with adult patients [9,10,11,12,13], with the exception of crizotinib [14, 15].
Most Phase I studies initially determined the pediatric dose by dividing the fixed adult dosage in milligrams by the average adult body surface area (BSA) of 1.7–1.8 m2, yielding a milligrams per square meter (mg/m²) dose advise [9, 10, 12]. However, not all differences in drug exposure in children compared to adults are due to differences in size, most often expressed as BSA and body weight (WT), but can also be caused by differences in body composition and function, including enzyme ontology [16,17,18]. It is therefore important to acknowledge these differences when making dose decisions in order to reduce bias in drug exposure. Several model-based methods have emerged over the last years to extrapolate pediatric PK from adults [16, 19, 20], where most focus have been on population PK (popPK) with allometric scaling and physiologically-based pharmacokinetic (PBPK) models [21]. Such models can inform (I) initial dose selection for PK studies and (II) study design in terms of sampling times and sample size based on the expected PK variability.
Population PK (popPK) models serve as mathematical tools to characterize drug exposure and primarily utilize a “top-down” approach by estimating parameters based on observed data from patient populations [19]. Allometric scaling constitutes a technique for the transformation of adult popPK models into pediatric counterparts, achieved through the application of a power exponent. Clearance parameters are typically scaled by a power exponent of 0.75, while a power exponent of 1 is used for volume parameters [22, 23].
PBPK models, on the other hand, adopt a “bottom-up” approach in which plasma and tissue concentration-time profiles can be predicted based on drug-specific characteristics, such as lipophilicity and molecular weight, without the need for human concentration measurements. PBPK models consist of a relatively large number of compartments that represent the different organs of the human body connected by blood flow [20]. These models are well recognized to possess translational utility by mechanistically depicting PK attributes and predicting exposure across diverse patient populations, facilitated by adapting anatomical and physiological aspects of the model to match the pediatric population [20]. For instance, in previous efforts a PBPK model for the TKI nilotinib was used to support the selection of a dose of 230 mg/m2 in children between the age of two and eighteen [24]. In earlier studies, allometric scaling has generally been found to perform adequately as compared to PBPK under two scenarios (i) a population older than 2 years for drugs with linear PK and (ii) for individuals younger than 2 years where popPK is possible but a maturation function is necessary [25,26,27]. The underlying reason being that the activity of enzymes are not fully developed in relation to size until approximately 2 years of age, necessitating the use of maturation functions to account for these developmental differences [25]. Therefore, predictions based solely on allometric scaling can lead to excessively high doses for children below 2 years.
The aim of this study was to evaluate the performance of popPK with allometric scaling and PBPK models in predicting the PK of TKIs for pediatric patients, based on adult models. For this purpose, previously published adult popPK models and the PBPK software PK-Sim were utilized. The drugs sunitinib, imatinib and pazopanib were selected as case studies due to their complex PK profiles, including large variability in exposure with impact on safety and efficacy, presence of active metabolites, time-varying clearance and non-linear absorption [28,29,30,31].
Methods
Literature search and data collection
A literature search was conducted within the PubMed database using the following search terms: “(sunitinib [Title/Abstract] OR imatinib [Title/Abstract] OR pazopanib [Title/Abstract]) AND (popPK [Title/Abstract] OR (population [Title/Abstract] AND pharmacokinetic [Title/Abstract]))”, (II) “(sunitinib[Title/Abstract] OR imatinib [Title/Abstract] OR pazopanib [Title/Abstract]) AND pharmacokinetic*[Title/Abstract]”, (III) “ (population [Title/Abstract] AND pharmacokinetic [Title/Abstract]) OR ((population [Title/Abstract] AND pharmacokinetic [Title/Abstract]) OR (popPK [Title/Abstract]))” AND pediatric* [Title/Abstract]”, (IV) “PBPK[Title/Abstract] AND pediatric*[Title/Abstract]” on May 2023. Relevant adult popPK publications were selected for potential use.
Adult and pediatric PK data were converted from the available figures into numerical values by the Webplotdigitizer (Version 4.4). Information regarding patient demographics, population size and drug administration protocols were derived from the corresponding publications to recreate the corresponding PK study as accurately as possible. Drug specific physicochemical properties were additionally collected and any missing values were derived from DrugBank, Pubchem, FDA and EMA chemistry reviews.
popPK models
Selection of the adult model
Adult popPK models for each of the three drugs was selected based on several criteria. For imatinib and sunitinib, the model had to describe the PK of both the parent drug and the active metabolite, preferably taking into account the correlations in clearance and volume of distribution. For imatinib, a model describing the increased clearance over time was required, whereas for pazopanib the model had to capture the saturation in drug (dose) absorption. Selected adult popPK models were translated into the R environment (version 4.1.0).
Translation of the adult model to the pediatric population
The pediatric PK simulations were conducted by scaling the adult popPK model parameters using allometric principles [23] (Eqs. 1 and 2).
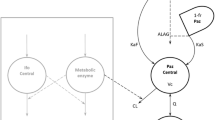
Here, the clearance and volume of distribution parameters are normalized by the individual weight of each child (WTi) by a reference value (70 kg) and scaled to the population parameters with exponents 0.75 (CLpediatric and Qpediatric) and 1 (Vcentral, pediatric and Vperipheral, pediatric), respectively. The absorption parameter remained unscaled. Simulations were performed using mrgsolve (version 0.11.2) in Rstudio (version 4.1.1.). A visual representation of the workflow can be found in Fig. 1.
PBPK models
Building the adult model
An adult PBPK model for each drug case was created within the software PK-Sim (version 9.1). The human body was represented by fifteen organ compartments - each divided into four sub-compartments consisting of plasma, endosomal, interstitial and intracellular space - connected by a systemic circulation [32]. To improve the extrapolation of PBPK models from adult to pediatric populations, the dependence on empirical adult-based PK estimations was minimized by including drug-specific physicochemical properties. Thereby, drug-specific mass-balance information, alongside enzyme maturation data, was integrated. Further details regarding the approach are provided in Sect. 2.3.2 to 2.3.4. If necessary, however, the drug specific properties were optimized to improve the model fit to the adult PK data. Priority was given to build a model that captured the drugs’ trough concentration (Ctrough), as this is used as a biomarker for drug efficacy [2].
The PBPK models were evaluated using data simulated from the adult popPK models. The decision to use simulated data instead of the observed data was prompted by the limitations of the available adult PK datasets, which were derived from a relatively small cohort and only presented as prediction-corrected values. The simulated datasets included 100 virtual individuals, and the patient covariate values were generated by resampling from the initial patient distributions. Sunitinib was administered at a daily dose of 50 mg for fourteen consecutive days. Imatinib was dosed at 400 mg daily for sixty consecutive days. For pazopanib, three different doses (200 mg, 400 mg, and 800 mg per day) were administered for fourteen consecutive days to assess the variations in bioavailability across the doses.
Absorption
To capture the process of absorption, information regarding tablet dissolution and the process of drug uptake through the gastro-intestinal tract were incorporated into the model. Firstly, drug dissolution and solubility profiles were obtained from the literature, with priority for values that corresponded to the acidity of the stomach environment (i.e. pH of 1.5 to 3.5). If the predictions were not consistent with the observations, the absorption rate from popPK models was used instead as a starting point. More complex absorption patterns were subsequently evaluated, including a Weibull function and the inclusion of a lag time. Different model descriptions of absorption were evaluated based on the simulated adult data, in conjunction to different partition coefficients and cellular permeability combinations (2.3.3. Distribution section). First-pass metabolism was taken into account via expression of enzymes and corresponding enzyme kinetics within the gastro-intestinal and hepatic tissue (2.3.4. Metabolism section).
Distribution
PK-Sim offers five methods to calculate the organ/plasma partition coefficient: Rodgers and Rowland (RR), PK-Sim standard, Poulin and Theil (PT), Schmitt and Berezhkovskiy, and three methods for cellular permeability: PK-Sim standard, charge dependent Schmitt and charge dependent Schmitt normalized to PK-Sim standard. In case none of the combinations gave a satisfactory model output as compared to the observed data, the drug lipophilicity (log(P)) was adjusted based on alternative literature values. Additionally, the model was informed about plasma protein binding, including the corresponding fraction unbound. Sunitinib is an acidic drug and typically binds to albumin [33], whereas imatinib is an alkaline drug that mainly binds to α1-acidglycoprotein [34]. Although pazopanib has alkaline properties it has been found to have a higher affinity for albumin [35].
Metabolism and elimination
Drug metabolism was incorporated into the model through the addition of Michaelis-Menten parameter values (i.e. Michaelis constant (Km) and maximum rate of reaction (Vmax)) for all relevant enzymes. If no Michaelis-Menten parameter values were available, the reported in vitro CL per mg of liver microsomes was used to enable enzyme-expression related scaling from adults to children.
To integrate enzyme-level drug metabolism with overall metabolism, PBPK models were enhanced by incorporating tissue- and age-specific protein expression data obtained from the Gene Expression Database on the Open Systems Pharmacology website. Three enzyme expression datasets were available: (1) whole genome expression arrays from ArrayExpress (ArrayExpress, 2010), (2) expressed sequence tags (EST) from UniGene and (3) RT-PCR (Reverse transcription polymerase chain reaction) derived gene expression estimates obtained from previous literature [36,37,38]. The performance of each enzyme expression dataset was evaluated in combination to the different available partition coefficients and cellular permeability calculation methods, yielding a total of 45 possible combinations.
Addition of renal and biliary clearance, both as an empirical clearance value (e.g. CLrenal) or more mechanistically through the expression of transporters with corresponding Michaelis-Menten parameter, were also assessed.
Translation of the adult model to the pediatric population
Once the adult PBPK model was created, the model was translated to a pediatric PBPK model by specifying the underlying age and weight of the population. Enzyme maturation adjustments were integrated using the PK-Sim Ontogeny database. A comprehensive explanation of the enzymes engaged in metabolizing each drug and their maturation patterns with age is available in Online Resource 1. Likewise, changes in anatomical and physiological features are automatically generated by the PK-Sim software. A visual representation of the workflow can be found in Fig. 1.
Evaluating model performance
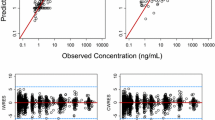
The final pediatric popPK and PBPK models were used to simulate PK observations that represent the studies of the observed pediatric data in terms of patient WT and age, drug dosing, sampling times, number of individuals and proportion of males versus females. In case of missing weights and BSA values, the reported World Health Organization (WHO) child growth standards were used for the corresponding age and sex [39]. For each model, the original dataset was re-simulated 200 times and summarized in a visual-predictive check (VPC) with the observed data [40].
Results
Literature search and data collection
A total of ten pediatric PK studies were identified (Table 1). Nine datasets were available for imatinib and four datasets for sunitinib. For pazopanib, only data from the tablet formulation were included, whereas the plasma concentration measurements from the suspension formulation were omitted. Unfortunately, the pazopanib measurements were not reported as observations, but only as mean and standard deviation for each distinct time points and dosage level. Therefore, to generate observations, ten data points were randomly sampled for each reported time point for each individual using the provided distribution.
Table 2 provides an overview of the physicochemical properties of each drug, as well as other relevant parameters related to drug absorption, distribution, metabolism, and elimination.
popPK models
Three popPK models were identified for sunitinib. The model by Yu et al. was selected as it captured the PK of both sunitinib and the active metabolite SU12662, including covariance between their parameters [29].
For imatinib the baseline model by Delbaldo et al. was selected, which described the PK of both imatinib as well as the active metabolite N-desmethyl-Imatinib (NDMI) on day 1 [41]. An increase in clearance in imatinib was added as a linear relative increase of 0.567% per day (giving rise to the reported average increase of 17% at day 30 [42], 34% at day 60 [43]), where time is a continuous variable:
For pazopanib, the model by Yu et al. [30] described the nonlinear absorption of the drug and different doses and was therefore selected. No further adjustments were made to the absorption structure, thus assuming that the dose-related absorption saturation occurred at the same absolute dose in children as in adults:
Where Emax (with a value of 1) represents the maximum effect of dose on bioavailability and ED50 (with a value of 480 mg) represents the dose level minus 200 mg at which the bioavailability is half. Allometric scaling of the clearance and distribution parameters was not part of the popPK models of imatinib and pazopanib and was therefore added for the pediatric simulations using a reference body weight of 70 kg.
PBPK models
Absorption and distribution
Since solubility and dissolution profiles did not give rise to the observed absorption profiles, a Weibull function was fitted for each drug, using the absorption rate constant from the popPK models as a starting point for the absorption half-life (Table 2). For pazopanib, the bioavailability at higher doses were overestimated despite informing the model on low tablet solubility. The evaluated adult (200, 400 and 800 mg) doses were therefore lowered proportionally to the bioavailability in the popPK model (Eq. 4), which resulted in adequate predictions of the observed data (Online Resource 1) [30]. For the pediatric population, the same proportional dose reduction was done using for the evaluated pediatric doses 275, 350, 450 and 600 mg/m2, assuming a BSA of 1 m2.
For all compounds, the partition coefficient, as calculated by the PK-Sim standard method, gave the best results. For imatinib, the initial logP of 1.99 [31] was increased to 4.0 to improve the model simulations, which is similar to the reported logP of 3.5 [44].
Metabolism and elimination
For sunitinib and imatinib, in vitro parameters of CYP metabolism, the Michaelis-Menten parameters were available for the parent compounds, but not for their active metabolites, SU12662 and NDMI. Therefore, the values for the parent compounds were employed for the metabolites. For pazopanib, no enzyme-specific values were available, and therefore, drug metabolism was incorporated through intrinsic clearance (Clint), which was based on drug clearance per total grams of CYP3A4 protein. The pazopanib CLint was optimized based on the observed data to 65 µl/min/mg microsomal protein, which is significantly lower than the reported optimized CLint of 175 µl/min/mg microsomal protein [45].
The PBPK simulations of the final adult models can be found in the Online Resource 1. For imatinib, the model captured the adult PK profile well, but had a tendency to underestimate the NMDI concentration-time profile. For sunitinib, the PBPK model underestimated maximum concentrations (Cmax), but captured Ctrough of the concentration-time profiles, particularly at steady state. For the active metabolite SU12662, the simulations underestimate the adult PK profiles. In case of pazopanib, the PBPK model captured the median PK profile well, but underestimated the population variability.
Pediatric simulations
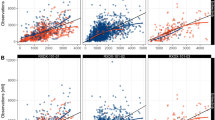
In the VPCs of imatinib, both computational models exhibited moderate overestimation of the Cmax and underestimation of the Ctrough in the median, the 2.5th and the 97.5th percentile in the 24-hour concentration profiles after first dose (Fig. 2a). At steady state, both models generally demonstrated accurate predictions of steady-state median Ctrough values, but underpredicted the steady-state 2.5th percentile. This is reflected back in the NMDI profiles, where both the PBPK and popPK models moderately overestimated the Cmax and underestimate the Ctrough for the median, the 2.5th and the 97.5th percentiles after the first dose (Fig. 2b). The steady-state Ctrough NMDI values was underpredicted by the PBPK model, whereas the popPK model captures the time-course but underestimates the variability. Both models overestimate the 2.5th percentile for NMDI concentration over time.
Visual predictive checks for the pediatric PBPK and popPK models. The solid black circles (•) represent observed drug concentrations. The dashed black lines (---) are the median, 10th and 90th percentiles of the observed data. The shaded areas correspond to the 95% confidence intervals for the median (red), 10th (blue) and 90th (blue) percentiles based on the simulated data (n = 200)
Both the PBPK and popPK models simulated concentration-time profiles that underpredicted the observed pediatric data over multiple dosing occasions of sunitinib, but captured the Ctrough at 28 days (672 h) (Fig. 2c). For the first 24-hour concentration profiles after first dose of sunitinib, the PBPK model tended to overestimate the initial profile, while the popPK model underpredicted the exposure, but both models effectively predicted steady-state Ctrough concentrations. For SU12662, the PBPK model generated concentrations markedly lower than the observed data, while the popPK model captured both the PK data but overestimated the variability (Fig. 2d). For the first 24-hour concentration profiles after first dose of sunitinib, the popPK model captures the full concentration-time profile of SU12662, whereas the PBPK model tends to overestimate the concentration at Cmax and underestimate the concentration at Ctrough.
In the case of pazopanib, simulations produced by the popPK and the PBPK model captured the observed data, but overestimated the 2.5th percentiles concentration observed during the first dose administration. Additionally, the PBPK model generally overestimated the concentrations at the 2.5th and 97.5th percentile (Fig. 2e).
The ratio of predicted Ctrough values after one dose and at steady-state and observed values are presented in Table 3. Using the threshold of 2-fold prediction error in exposure, the PBPK model underestimated the Ctrough for active metabolites NMDI and SU12662.
Discussion
This investigation evaluated the performance of popPK models with allometric scaling and PBPK models in predicting the PK of TKIs for pediatric patients, using three TKIs with intricate PK characteristics and administered to children across diverse age and dosing ranges. Allometric scaling of popPK models predicted exposure within 0.5-2 fold the observed values for all TKIs. On the other hand, PBPK-based predictions underpredicted the exposure of the active metabolites, thus resulting in potentially larger miscalculations of initial dose selection.
For imatinib, both the popPK and PBPK models tended to overestimate the Cmax of children in the first 0–24 h time profiles, particularly for the active metabolite NMDI, but captured the Ctrough values adequately (Fig. 2). This could potentially be due to differences in the absorption rate between adults and children that are not well represented by both models, although the reported duration of absorption estimated for children resembled that of adults at 1.67 [46] and 1.5 h [47], respectively. Regarding sunitinib, both models successfully simulated PK profiles that resemble the observed data; however, the PBPK model generated a higher Cmax in the 24-hour profile (Fig. 2). This phenomenon is, however, already witnessed in the simulations arising from the adult PBPK model compared to the observed data, and therefore regarded as a constraint within the PBPK model rather than an issue of extrapolation. Similarly, the PBPK projections for the active metabolite SU12662 were initially lower than the observed data in adults, indicating that underestimation of the pediatric data is not related to inadequate extrapolation. Regarding pazopanib, the PBPK model demonstrated an overestimation of observed pediatric concentrations in the 2.5th and 95th percentile (Fig. 2), potentially stemming from an overestimated bioavailability at elevated doses. This might be linked to an overestimation of the absorbed fraction from the gastrointestinal tract in children, considering the low first-pass effect for pazopanib [48].
As highlighted in the introduction section, model-based predictions could be employed to (I) inform first dose selection [49, 50]. For the Phase I PK studies of TKIs, a maximum of 2-fold deviation from the adult exposure was deemed acceptable [9,10,11,12,13]. Based on this limit, the Ctrough PK predictions were considered acceptable for the popPK model whereas in 2 out of 5 cases the PBPK predictions were outside the 2-fold range (Table 3). Therefore, while previous research has demonstrated that both allometric scaling and PBPK models produced predictions within the accepted prediction error for children (≥ 2 years) based on data from adults [21, 25, 26, 49, 51, 52], our findings suggest that extrapolations based on allometric scaling might perform better for compounds with complex PK. However, considering that our evaluation focused on only three compounds out of > 80 TKIs that have received regulatory approval [53], the generalizability of our findings to all available TKIs remains limited. Additionally, it should be noted that a 2-fold prediction error remains large for TKIs, in which exposure is crucial for drug efficacy and safety and thus a lower deviation is desired [2, 28]. Based on the results for pazopanib, the PBPK model overpredicted the concentration by 50%, whereas the popPK model had an overprediction of 10%. Therefore, it is anticipated that PBPK recommendations would suggest lower dose for evaluation in a pediatric PK study as compared to those derived from the popPK model.
An additional purpose of predictions based on models is to (II) inform study design [49, 50]. Given that drugs like imatinib, sunitinib, and pazopanib have demonstrated considerable variability in exposure levels among different patients [28], this variability can impact the necessary sample size for a pediatric PK study focused on achieving comparable exposure levels [5]. Hence, it is imperative that model-based predictions capture the variability within PK profiles, a requirement that both PBPK and popPK models have largely fulfilled in the context of this investigation. Given that the principal objective of forecasting pediatric PK profiles is to facilitate informed dose selection, such computational forecasts can serve as a valuable instrument within this decision-making process [49].
In addition to the comparison between simulated and observed profiles, there exist various pragmatic considerations to account for when selecting PBPK and popPK models. Both the PBPK and popPK methodologies rely upon the availability of adult-centric data, however, the PBPK model necessitating supplementary in vitro data to facilitate model generation. Furthermore, the capacity of PBPK models is constrained by software limitations. Consequently, if the essential features are absent within the PBPK software, it might be more feasible to integrate mechanistic components within a popPK model. In the context of TKIs, it is anticipated that factors such as the type of disease and the extent of tumor burden could influence PK. While the concept of “physiologically-based” underscores the capability to extrapolate adult anatomical and physiological attributes into pediatric models, if pivotal structural elements are deficient within the model or if the pathways of elimination are inadequately established the translational capacity might not exceed that of a popPK approach [21].
There exist several limitations within this study that merit emphasis. Concerning the PBPK models, the incorporation of time-dependent clearance and dose-dependent bioavailability was added empirically due to inherent software constraints, thereby relying on the assumption that there was concordance between pediatric and adult subjects. Notably, for pazopanib, this approach led to an overestimation of the higher concentrations. A PBPK software that accommodates mechanistic integration of dose-dependent bioavailability is anticipated to enhance model predictability. Furthermore, due to the absence of individual-level data, the differentiation between age and dose categories was unfeasible during simulations, thereby precluding subgroup analyses aimed at identifying factors that could have influenced model misrepresentation.
In summary, based on the studied TKI cases, it appears that allometric scaling of popPK models could be more suitable for predicting exposure in pediatric patients aged 2 years and older. This is likely due to the model’s ability to account for TKIs with complex PK characteristics. Given the limited amount of drug cases, however, the generalizability of our findings to all available TKIs remains limited. One potential strategy to address the existing uncertainty in model-guided TKI exposure projections is to use both methods simultaneously, to evaluate if discrepancies arise in projections for specific compounds. In such instances, an additional investigation could be performed to identify the origins of discrepancies in order to understand how they lead to distinct projections between the two approaches.
Data availability
No datasets were generated or analysed during the current study.
Code availability
The code used for data analysis and statistical modeling is available upon request from the corresponding author L.E.F..
References
Kyu HH, Stein CE, Boschi Pinto C et al (2018) Causes of death among children aged 5–14 years in the WHO European Region: a systematic analysis for the global burden of Disease Study 2016. Lancet Child Adolesc Heal 2:321–337. https://doi.org/10.1016/S2352-4642(18)30095-6
Janssen JM, Dorlo TPC, Steeghs N et al (2020) Pharmacokinetic targets for therapeutic drug monitoring of small molecule kinase inhibitors in Pediatric Oncology. Clin Pharmacol Ther 108:494–505. https://doi.org/10.1002/cpt.1808
Kaczmarska A, Śliwa P, Lejman M, Zawitkowska J (2021) The use of inhibitors of tyrosine kinase in Paediatric Haemato-Oncology-when and why? Int J Mol Sci 22. https://doi.org/10.3390/ijms222112089
Verschuur AC, Bajčiová V, Mascarenhas L et al (2019) Sunitinib in pediatric patients with advanced gastrointestinal stromal tumor: results from a phase I/II trial. Cancer Chemother Pharmacol 84:41–50. https://doi.org/10.1007/s00280-019-03814-5
EMA (2018) Reflection paper on the use of extrapolation in the development of medicines for paediatrics
EMA/CHMP (2022) Structured guidance on the use of extrapolation
Burckart G, Pediatric Extrapolation (2023) Jul in FDA Submissions – Sources of Data. https://www.ema.europa.eu/en/documents/presentation/presentation-paediatric-extrapolation-fda-submissions-sources-data-g-burckart_en.pdf. Accessed 30
Mulugeta L, Experience in FDA Submissions with Matching Pediatric Drug Exposure to Adult Drug Exposure. https://www.pharmacy.umaryland.edu/media/SOP/wwwpharmacyumarylandedu/centers/cersievents/pediatricpbpk/Mulugeta-exposure matching in FDA submissions_final2.pdf. Accessed 30 Jul 2023
Champagne MA, Capdeville R, Krailo M et al (2004) Imatinib mesylate (STI571) for treatment of children with Philadelphia chromosome-positive leukemia: results from a Children’s Oncology Group phase 1 study. Blood 104:2655–2660. https://doi.org/10.1182/blood-2003-09-3032
Zwaan CM, Rizzari C, Mechinaud F et al (2013) Dasatinib in Children and adolescents with relapsed or refractory leukemia: results of the CA180-018 phase I dose-escalation study of the innovative therapies for children with Cancer Consortium. J Clin Oncol 31:2460–2468. https://doi.org/10.1200/JCO.2012.46.8280
Hijiya N, Zwaan CM, Rizzari C et al (2020) Pharmacokinetics of Nilotinib in Pediatric patients with Philadelphia chromosome–positive chronic myeloid leukemia or Acute Lymphoblastic Leukemia. Clin Cancer Res 26:812–820. https://doi.org/10.1158/1078-0432.CCR-19-0090
Widemann BC, Kim A, Fox E et al (2012) A phase I trial and pharmacokinetic study of sorafenib in children with refractory solid tumors or leukemias: a children’s Oncology Group Phase I Consortium report. Clin cancer Res off J Am Assoc Cancer Res 18:6011–6022. https://doi.org/10.1158/1078-0432.CCR-11-3284
Meneses-Lorente G, Guerini E, Mercier F et al (2023) Entrectinib dose confirmation in pediatric oncology patients: pharmacokinetic considerations. Cancer Chemother Pharmacol 91:239–246. https://doi.org/10.1007/s00280-023-04510-1
Balis FM, Thompson PA, Mosse YP et al (2017) First-dose and steady-state pharmacokinetics of orally administered crizotinib in children with solid tumors: a report on ADVL0912 from the Children’s Oncology Group Phase 1/Pilot Consortium. Cancer Chemother Pharmacol 79:181–187. https://doi.org/10.1007/s00280-016-3220-6
Mossé YP, Lim MS, Voss SD et al (2013) Safety and activity of crizotinib for paediatric patients with refractory solid tumours or anaplastic large-cell lymphoma: a Children’s Oncology Group phase 1 consortium study. Lancet Oncol 14:472–480. https://doi.org/10.1016/S1470-2045(13)70095-0
De Cock RFW, Piana C, Krekels EHJ et al (2011) The role of population PK-PD modelling in paediatric clinical research. Eur J Clin Pharmacol 67 Suppl 1:5–16. https://doi.org/10.1007/s00228-009-0782-9
Wang K, Jiang K, Wei X et al (2021) Physiologically based pharmacokinetic models are effective support for Pediatric Drug Development. AAPS PharmSciTech 22:208. https://doi.org/10.1208/s12249-021-02076-w
Calvier EAM, Krekels EHJ, Välitalo PAJ et al (2017) Allometric scaling of Clearance in Paediatric patients: when does the magic of 0.75 fade? Clin Pharmacokinet 56:273–285. https://doi.org/10.1007/s40262-016-0436-x
Mould DR, Upton RN (2013) Basic concepts in Population modeling, Simulation, and model-based Drug Development—Part 2: introduction to Pharmacokinetic modeling methods. CPT Pharmacometrics Syst Pharmacol 2:38. https://doi.org/10.1038/psp.2013.14
Jones HM, Rowland-Yeo K (2013) Basic concepts in physiologically based pharmacokinetic modeling in Drug Discovery and Development. CPT Pharmacometrics Syst Pharmacol 2:63. https://doi.org/10.1038/psp.2013.41
Mahmood I, Tegenge MA (2019) A comparative study between Allometric Scaling and physiologically based pharmacokinetic modeling for the prediction of drug Clearance from neonates to adolescents. J Clin Pharmacol 59:189–197. https://doi.org/10.1002/jcph.1310
Holford N, Heo Y-A, Anderson B (2013) A Pharmacokinetic Standard for Babies and adults. J Pharm Sci 102:2941–2952. https://doi.org/10.1002/jps.23574
Anderson BJ, Holford NHG (2009) Mechanistic basis of using body size and maturation to Predict Clearance in humans. Drug Metab Pharmacokinet 24:25–36. https://doi.org/10.2133/dmpk.24.25
Heimbach T, Lin W, Hourcade-Potelleret F et al (2019) Physiologically based pharmacokinetic modeling to supplement Nilotinib Pharmacokinetics and confirm dose selection in Pediatric patients. J Pharm Sci 108:2191–2198. https://doi.org/10.1016/j.xphs.2019.01.028
Krekels EHJ, Calvier EAM, van der Graaf PH, Knibbe CAJ (2019) Children are not small adults, but can we treat them as such? CPT Pharmacometrics Syst Pharmacol 8:34–38. https://doi.org/10.1002/psp4.12366
Wu Q, Peters SA (2019) A retrospective evaluation of Allometry, Population Pharmacokinetics, and physiologically-based pharmacokinetics for Pediatric Dosing using clearance as a surrogate. CPT Pharmacometrics Syst Pharmacol 8:220–229. https://doi.org/10.1002/psp4.12385
Ke APBPK (2023) Jul modeling and allometric scaling in pediatric drug development: where do we draw the line? https://cersi.umd.edu/sites/cersi.umd.edu/files/Day 1 Slide Set %281%29-1-83.pdf. Accessed 30
Lankheet NAG, Desar IME, Mulder SF et al (2017) Optimizing the dose in cancer patients treated with imatinib, sunitinib and pazopanib. Br J Clin Pharmacol 83:2195–2204. https://doi.org/10.1111/bcp.13327
Yu H, Steeghs N, Kloth JSL et al (2015) Integrated semi-physiological pharmacokinetic model for both sunitinib and its active metabolite SU12662. Br J Clin Pharmacol 79:809–819. https://doi.org/10.1111/bcp.12550
Yu H, van Erp N, Bins S et al (2017) Development of a pharmacokinetic model to describe the Complex Pharmacokinetics of Pazopanib in Cancer patients. Clin Pharmacokinet 56:293–303. https://doi.org/10.1007/s40262-016-0443-y
Peng B, Lloyd P, Schran H (2005) Clinical pharmacokinetics of Imatinib. Clin Pharmacokinet 44:879–894. https://doi.org/10.2165/00003088-200544090-00001
Willmann S, Lippert J, Sevestre M et al (2003) PK-Sim®: a physiologically based pharmacokinetic ‘whole-body’ model. BIOSILICO 1:121–124. https://doi.org/10.1016/S1478-5382(03)02342-4
Kabir MZ, Tee W-V, Mohamad SB et al (2017) Comprehensive insight into the binding of sunitinib, a multi-targeted anticancer drug to human serum albumin. Spectrochim Acta Part Mol Biomol Spectrosc 181:254–263. https://doi.org/10.1016/j.saa.2017.03.059
Adiwidjaja J, Boddy AV, McLachlan AJ (2020) Implementation of a physiologically based pharmacokinetic modeling Approach to Guide Optimal Dosing regimens for Imatinib and potential drug interactions in Paediatrics. Front Pharmacol 10
Imbs D-C, Paludetto M-N, Négrier S et al (2016) Determination of unbound fraction of pazopanib in vitro and in cancer patients reveals albumin as the main binding site. Invest New Drugs 34:41–48. https://doi.org/10.1007/s10637-015-0304-9
Nishimura M, Yaguti H, Yoshitsugu H et al (2003) Tissue distribution of mRNA expression of human cytochrome P450 isoforms assessed by high-sensitivity real-time reverse transcription PCR. Yakugaku Zasshi 123:369–375. https://doi.org/10.1248/yakushi.123.369
Nishimura M, Naito S (2005) Tissue-specific mRNA expression profiles of human ATP-binding cassette and solute carrier transporter superfamilies. Drug Metab Pharmacokinet 20:452–477. https://doi.org/10.2133/dmpk.20.452
Nishimura M, Naito S (2006) Tissue-specific mRNA expression profiles of human phase I metabolizing enzymes except for cytochrome P450 and phase II metabolizing enzymes. Drug Metab Pharmacokinet 21:357–374. https://doi.org/10.2133/dmpk.21.357
Organization WH (2006) WHO child growth standards: length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age: methods and development
Bergstrand M, Hooker AC, Wallin JE, Karlsson MO (2011) Prediction-corrected visual predictive checks for diagnosing nonlinear mixed-effects models. AAPS J 13:143–151. https://doi.org/10.1208/s12248-011-9255-z
Delbaldo C, Chatelut E, Ré M et al (2006) Pharmacokinetic-pharmacodynamic relationships of imatinib and its main metabolite in patients with advanced gastrointestinal stromal tumors. Clin cancer Res off J Am Assoc Cancer Res 12:6073–6078. https://doi.org/10.1158/1078-0432.CCR-05-2596
Demetri GD, Wang Y, Wehrle E et al (2009) Imatinib plasma levels are correlated with clinical benefit in patients with unresectable/metastatic gastrointestinal stromal tumors. J Clin Oncol off J Am Soc Clin Oncol 27:3141–3147. https://doi.org/10.1200/JCO.2008.20.4818
Judson I, Ma P, Peng B et al (2005) Imatinib pharmacokinetics in patients with gastrointestinal stromal tumour: a retrospective population pharmacokinetic study over time. EORTC Soft tissue and Bone Sarcoma Group. Cancer Chemother Pharmacol 55:379–386. https://doi.org/10.1007/s00280-004-0876-0
PubChem, Imatinib Compound Summary. https://pubchem.ncbi.nlm.nih.gov/compound/Imatinib#section=Chemical-and-Physical-Properties. Accessed 30 Jul 2023
Fink C, Sun D, Wagner K et al (2020) Evaluating the role of solubility in oral absorption of Poorly Water-Soluble drugs using physiologically-based pharmacokinetic modeling. Clin Pharmacol Ther 107:650–661. https://doi.org/10.1002/cpt.1672
Menon-Andersen D, Mondick JT, Jayaraman B et al (2009) Population pharmacokinetics of imatinib mesylate and its metabolite in children and young adults. Cancer Chemother Pharmacol 63:229–238. https://doi.org/10.1007/s00280-008-0730-x
Schmidli H, Peng B, Riviere G-J et al (2005) Population pharmacokinetics of imatinib mesylate in patients with chronic-phase chronic myeloid leukaemia: results of a phase III study. Br J Clin Pharmacol 60:35–44. https://doi.org/10.1111/j.1365-2125.2005.02372.x
Verheijen RB, Beijnen JH, Schellens JHM et al (2017) Clinical pharmacokinetics and pharmacodynamics of Pazopanib: towards optimized dosing. Clin Pharmacokinet 56:987–997. https://doi.org/10.1007/s40262-017-0510-z
Johnson TN, Rostami-Hodjegan A, Tucker GT (2006) Prediction of the Clearance of Eleven Drugs and Associated Variability in neonates, infants and children. Clin Pharmacokinet 45:931–956. https://doi.org/10.2165/00003088-200645090-00005
Sheiner LB (1997) Learning versus confirming in clinical drug development. Clin Pharmacol Ther 61:275–291. https://doi.org/10.1016/S0009-9236(97)90160-0
Mansoor N, Ahmad T, Alam Khan R et al (2019) Prediction of Clearance and Dose of Midazolam in Preterm and Term neonates: a comparative study between Allometric Scaling and physiologically based pharmacokinetic modeling. Am J Ther 26:e32–e37. https://doi.org/10.1097/MJT.0000000000000506
Malik PRV, Edginton AN (2019) Physiologically-based pharmacokinetic modeling vs. allometric scaling for the prediction of Infliximab Pharmacokinetics in Pediatric patients. CPT Pharmacometrics Syst Pharmacol 8:835–844. https://doi.org/10.1002/psp4.12456
Centanni M, Thijs A, Desar I et al (2022) Optimization of blood pressure measurement practices for pharmacodynamic analyses of tyrosine-kinase inhibitors. https://doi.org/10.1111/cts.13423. Clin Transl Sci n/a
Marangon E, Citterio M, Sala F et al (2009) Pharmacokinetic profile of imatinib mesylate and N-desmethyl-imatinib (CGP 74588) in children with newly diagnosed Ph + acute leukemias. Cancer Chemother Pharmacol 63:563–566. https://doi.org/10.1007/s00280-008-0764-0
Petain A, Kattygnarath D, Azard J et al (2008) Population pharmacokinetics and pharmacogenetics of imatinib in children and adults. Clin cancer Res off J Am Assoc Cancer Res 14:7102–7109. https://doi.org/10.1158/1078-0432.CCR-08-0950
Glade Bender JL, Lee A, Reid JM et al (2013) Phase I pharmacokinetic and pharmacodynamic study of pazopanib in children with soft tissue sarcoma and other refractory solid tumors: a children’s oncology group phase I consortium report. J Clin Oncol off J Am Soc Clin Oncol 31:3034–3043. https://doi.org/10.1200/JCO.2012.47.0914
Wang E, DuBois SG, Wetmore C, Khosravan R (2020) Population pharmacokinetics-pharmacodynamics of sunitinib in pediatric patients with solid tumors. Cancer Chemother Pharmacol 86:181–192. https://doi.org/10.1007/s00280-020-04106-z
Yu Y, DuBois SG, Wetmore C, Khosravan R (2020) Physiologically based pharmacokinetic modeling and Simulation of Sunitinib in Pediatrics. AAPS J 22:31. https://doi.org/10.1208/s12248-020-0423-x
Wagner C, Kesisoglou F, Pepin XJH et al (2021) Use of physiologically based pharmacokinetic modeling for Predicting Drug-Food interactions: recommendations for improving predictive performance of low confidence Food Effect models. AAPS J 23:85. https://doi.org/10.1208/s12248-021-00601-0
(2012) Oligoarylamines, Oligoarylamides, Oligoarylcarbamates, and Oligoarylureas. In: Lead Optimization for Medicinal Chemists. pp 365–376
Information NC (2023) for B NPubChem Compound Summary for CID 5329102, Sunitinib. https://pubchem.ncbi.nlm.nih.gov/compound/Sunitinib. Accessed 2 Dec 2023
(2023) Drugbank online - Imatinib. https://go.drugbank.com/drugs/DB00619. Accessed 2 Dec 2023
(2023) Drugbank online - Pazopanib hydrochloride. https://go.drugbank.com/salts/DBSALT000135. Accessed 2 Dec 2023
Sugiyama M, Fujita K, Murayama N et al (2011) Sorafenib and Sunitinib, two anticancer drugs, inhibit CYP3A4-Mediated and activate CY3A5-Mediated midazolam 1′-Hydroxylation. Drug Metab Dispos 39 :757 LP – 762. https://doi.org/10.1124/dmd.110.037853
Funding
Open access funding provided by Uppsala University. The Swedish Cancer Society (CAN 20 1226 PjF). The Swedish Childhood Cancer Fund (PR2021-0064).
Author information
Authors and Affiliations
Contributions
M.C, L.E.F., M.O.K. wrote the manuscript and all authors commented on the final version of the manuscript; M.C., L.E.F and M.O.K. designed the research and analyzed the data; M.C., O.Z. and D.E. performed the research.
Corresponding author
Ethics declarations
Competing interests
M.C, L.E.F., M.O.K., O.Z., and D.E. declare no competing interests for this work.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Centanni, M., Zaher, O., Elhad, D. et al. Physiologically-based pharmacokinetic models versus allometric scaling for prediction of tyrosine-kinase inhibitor exposure from adults to children. Cancer Chemother Pharmacol (2024). https://doi.org/10.1007/s00280-024-04678-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00280-024-04678-0