Abstract
Background
Camrelizumab combined with chemotherapy is approved across tumor types. However, only a fraction of patients benefits from immunotherapy, and biomarkers such as the expression of PD-L1, tumor mutational burden, and CXCL11 are expensive and suboptimal specificity for cancer patients. An exposure–response (E–R) relationship has been reported in many immune checkpoint inhibitors (ICIs), and the trough concentrations and other drug exposure metrics are broadly used to guide dosing decisions, assess exposure–outcomes relationships, and ultimately predict outcomes based on those relationships. However, the potential use of trough concentration levels for camrelizumab is still not clear.
Methods
Blood samples were obtained at trough levels after doses 3 and 4 from 77 patients with advanced lung cancer who received camrelizumab (200 mg Q3 W) monotherapy or combined with chemotherapy. We optimized a competitive ELISA method to measure the trough concentration.
Results
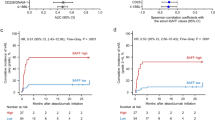
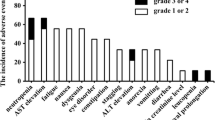
We found that the trough concentration was steady after 3 dose cycles, and the trough concentration level of camrelizumab was higher in patients who developed immune-related adverse effects (irAEs) than in those who did not (P < 0.05) but was not observed in disease progression and PFS (P > 0.05). Age (< 65 years old), no smoking history, and efficacy evaluation after 4-dose treatment were associated with PFS (P < 0.05), but no significance was observed in other clinical characteristics. Total bilirubin and albumin had an influence on trough concentration, and monocytes and albumin were independent risk factors for PFS (P < 0.05).
Conclusions
Our results suggest that the trough concentration level of camrelizumab might be a risk factor for the occurrence of irAEs in advanced lung cancer, and using the immunotherapy as early as possible may bring better clinical outcomes.



Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Tison A, Quéré G, Misery L, Funck-Brentano E, Danlos FX, Routier E, Robert C, Loriot Y, Lambotte O, Bonniaud B, Scalbert C, Maanaoui S, Lesimple T, Martinez S, Marcq M, Chouaid C, Dubos C, Brunet-Possenti F, Stavris C, Chiche L, Beneton N, Mansard S, Guisier F, Doubre H, Skowron F, Aubin F, Zehou O, Roge C, Lambert M, Pham-Ledard A, Beylot-Barry M, Veillon R, Kramkimel N, Giacchero D, De Quatrebarbes J, Michel C, Auliac JB, Gonzales G, Decroisette C, Le Garff G, Carpiuc I, Vallerand H, Nowak E, Cornec D, Kostine M (2019) Safety and efficacy of immune checkpoint inhibitors in patients with cancer and preexisting autoimmune disease: a nationwide, multicenter cohort study, arthritis. Rheumatol 71:2100–2111
Emens LA, Ascierto PA, Darcy PK, Demaria S, Eggermont AMM, Redmond WL, Seliger B, Marincola FM (2017) Cancer immunotherapy: opportunities and challenges in the rapidly evolving clinical landscape. Eur J Cancer 81:116–129
Yang J, Zhao H, Garnett C, Rahman A, Gobburu JV, Pierce W, Schechter G, Summers J, Keegan P, Booth B, Wang Y (2013) The combination of exposure-response and case-control analyses in regulatory decision making. J Clin Pharmacol 53:160–166
Dai HI, Vugmeyster Y, Mangal N (2020) Characterizing exposure-response relationship for therapeutic monoclonal antibodies in immuno-oncology and beyond: challenges, perspectives, and prospects. Clin Pharmacol Ther 108:1156–1170
Basak EA, Koolen SLW, Hurkmans DP, Schreurs MWJ, Bins S, Oomen-de Hoop E, Wijkhuijs AJM, Besten ID, Sleijfer S, Debets R, van der Veldt AAM, Aerts J, Mathijssen RHJ (2019) Correlation between nivolumab exposure and treatment outcomes in non-small-cell lung cancer. Eur J Cancer 109:12–20
Hurkmans DP, Basak EA, van Dijk T, Mercieca D, Schreurs MWJ, Wijkhuijs AJM, Bins S, Hoop EO, Debets R, Joerger M, Odink A, van der Veldt AAM, van der Leest CH, Aerts J, Mathijssen RHJ, Koolen SLW (2019) A prospective cohort study on the pharmacokinetics of nivolumab in metastatic non-small cell lung cancer, melanoma, and renal cell cancer patients. J Immunother Cancer 7:192
Turner DC, Kondic AG, Anderson KM, Robinson AG, Garon EB, Riess JW, Jain L, Mayawala K, Kang J, Ebbinghaus SW, Sinha V, de Alwis DP, Stone JA (2018) Pembrolizumab exposure-response assessments challenged by association of cancer cachexia and catabolic clearance. Clin Cancer Res 24:5841–5849
Li H, Yu J, Liu C, Liu J, Subramaniam S, Zhao H, Blumenthal GM, Turner DC, Li C, Ahamadi M, de Greef R, Chatterjee M, Kondic AG, Stone JA, Booth BP, Keegan P, Rahman A, Wang Y (2017) Time dependent pharmacokinetics of pembrolizumab in patients with solid tumor and its correlation with best overall response. J Pharmacokinet Pharmacodyn 44:403–414
Koguchi Y, Iwamoto N, Shimada T, Chang SC, Cha J, Curti BD, Urba WJ, Piening BD, Redmond WL (2021) Trough levels of ipilimumab in serum as a potential biomarker of clinical outcomes for patients with advanced melanoma after treatment with ipilimumab. J Immunother Cancer 9:10
Wilkins JJ, Brockhaus B, Dai H, Vugmeyster Y, White JT, Brar S, Bello CL, Neuteboom B, Wade JR, Girard P, Khandelwal A (2019) Time-varying clearance and impact of disease state on the pharmacokinetics of avelumab in Merkel cell carcinoma and urothelial carcinoma. CPT Pharmacometrics Syst Pharmacol 8:415–427
Wang CY, Sheng CC, Ma GL, Xu D, Liu XQ, Wang YY, Zhang L, Cui CL, Xu BH, Song YQ, Zhu J, Jiao Z (2021) Population pharmacokinetics of the anti-PD-1 antibody camrelizumab in patients with multiple tumor types and model-informed dosing strategy. Acta Pharmacol Sin 42:1368–1375
Huang J, Xu B, Mo H, Zhang W, Chen X, Wu D, Qu D, Wang X, Lan B, Yang B, Wang P, Zhang H, Yang Q, Jiao Y (2018) Safety, activity, and biomarkers of SHR-1210, an anti-PD-1 antibody, for patients with advanced esophageal carcinoma. Clin Cancer Res 24:1296–1304
Feng Y, Wang X, Bajaj G, Agrawal S, Bello A, Lestini B, Finckenstein FG, Park JS, Roy A (2017) Nivolumab exposure-response analyses of efficacy and safety in previously treated squamous or nonsquamous non-small cell lung cancer. Clin Cancer Res 23:5394–5405
Dreesen E, Bossuyt P, Mulleman D, Gils A, Pascual-Salcedo D (2017) Practical recommendations for the use of therapeutic drug monitoring of biopharmaceuticals in inflammatory diseases. Clin Pharmacol Adv Appl 9:101–111
Vermeire S, Dreesen E, Papamichael K, Dubinsky MC (2020) How, when, and for whom should we perform therapeutic drug monitoring? Clin Gastroenterol Hepatol Off Clin Practice J Am Gastroenterol Assoc 18:1291–1299
Lala M, Li TR, de Alwis DP, Sinha V, Mayawala K, Yamamoto N, Siu LL, Chartash E, Aboshady H, Jain L (2020) A six-weekly dosing schedule for pembrolizumab in patients with cancer based on evaluation using modelling and simulation. Eur J Cancer 131:68–75
Markham A, Keam SJ (2019) Camrelizumab: first global approval. Drugs 79:1355–1361
Mo H, Huang J, Xu J, Chen X, Wu D, Qu D, Wang X, Lan B, Wang X, Xu J, Zhang H, Chi Y, Yang Q, Xu B (2018) Safety, anti-tumour activity, and pharmacokinetics of fixed-dose SHR-1210, an anti-PD-1 antibody in advanced solid tumours: a dose-escalation, phase 1 study. Br J Cancer 119:538–545
Coss CC, Clinton SK, Phelps MA (2018) Cachectic cancer patients: immune to checkpoint inhibitor therapy? Clin Cancer Res 24:5787–5789
Flint TR, Janowitz T, Connell CM, Roberts EW, Denton AE, Coll AP, Jodrell DI, Fearon DT (2016) Tumor-induced IL-6 reprograms host metabolism to suppress anti-tumor immunity. Cell Metab 24:672–684
Baverel P, Roskos L, Tatipalli M, Lee N, Stockman P, Taboada M, Vicini P, Horgan K, Narwal R (2019) Exposure-response analysis of overall survival for tremelimumab in unresectable malignant mesothelioma: the confounding effect of disease status. Clin Transl Sci 12:450–458
Feng Y, Roy A, Masson E, Chen TT, Humphrey R, Weber JS (2013) Exposure-response relationships of the efficacy and safety of ipilimumab in patients with advanced melanoma. Clin Cancer Res 19:3977–3986
Badawi M, Coss CC, Phelps MA (2019) Letter to the Editor: Exposure-response or clearance-response relationship in immune checkpoint therapy?-A comment on “correlation between nivolumab exposure and treatment outcomes in non-small-cell lung cancer” by Basak et al. Eur J Cancer 114:25–26
Kawakatsu S, Bruno R, Kågedal M, Li C, Girish S, Joshi A, Wu B (2021) Confounding factors in exposure-response analyses and mitigation strategies for monoclonal antibodies in oncology. Br J Clin Pharmacol 87:2493–2501
Pyzik M, Rath T, Lencer WI, Baker K, Blumberg RS (2015) FcRn: the architect behind the immune and nonimmune functions of IgG and albumin. J Immunol (Baltimore MD: 1950) 194:4595–4603
Dirks NL, Meibohm B (2010) Population pharmacokinetics of therapeutic monoclonal antibodies. Clin Pharmacokinet 49:633–659
Huang L, Li L, Zhou Y, Yang Z, Wang M, Gao Y, Yang Y, Yang F, Liu B, Hong X, Chen G (2020) Clinical characteristics correlate with outcomes of immunotherapy in advanced non-small cell lung cancer. J Cancer 11:7137–7145
Araujo JM, Prado A, Cardenas NK, Zaharia M, Dyer R, Doimi F, Bravo L, Pinillos L, Morante Z, Aguilar A, Mas LA, Gomez HL, Vallejos CS, Rolfo C, Pinto JA (2016) Repeated observation of immune gene sets enrichment in women with non-small cell lung cancer. Oncotarget 7:20282–20292
Shien K, Papadimitrakopoulou VA (2016) Wistuba, II, Predictive biomarkers of response to PD-1/PD-L1 immune checkpoint inhibitors in non-small cell lung cancer. Lung cancer (Amsterdam, Netherlands) 99:79–87
Li C, Li C, Zhi C, Liang W, Wang X, Chen X, Lv T, Shen Q, Song Y, Lin D, Liu H (2019) Clinical significance of PD-L1 expression in serum-derived exosomes in NSCLC patients. J Transl Med 17:355
Wang X, Ricciuti B, Nguyen T, Li X, Rabin MS, Awad MM, Lin X, Johnson BE, Christiani DC (2021) Association between smoking history and tumor mutation burden in advanced non-small cell lung cancer. Can Res 81:2566–2573
Lobefaro R, Viscardi G, Di Liello R, Massa G, Iacovino ML, Sparano F, Della Corte CM, Ferrara R, Signorelli D, Proto C, Prelaj A, Galli G, De Toma A, Brambilla M, Ganzinelli M, Trevisan B, Ciardiello F, De Braud F, Morgillo F, Garassino MC, Lo Russo G (2021) Immunotherapy in advanced non-small cell lung cancer patients with poor performance status: the role of clinical-pathological variables and inflammatory biomarkers. Lung cancer (Amsterdam, Netherlands) 152:165–173
Pan ZK, Ye F, Wu X, An HX, Wu JX (2015) Clinicopathological and prognostic significance of programmed cell death ligand1 (PD-L1) expression in patients with non-small cell lung cancer: a meta-analysis. J Thorac Dis 7:462–470
Acknowledgements
This work was supported by the Project of Beijing Medical Award Foundation (YXJL-2022-0187-0013), Beijing Hongdingxiang Public Welfare Development Center (BJ-HDX-20220437) and The General Program of Harbin Medical University Cancer Hospital Haiyan Fund (JJMS2022-19). The authors are grateful to the patients and their families for their participation in this study. The study was approved by the Ethics Committee of Harbin Medical University Cancer Hospital (KY2022-48).
Author information
Authors and Affiliations
Contributions
MD and SJ: designed the study and wrote the article. MC, FY and YY: performed the experiment. Blood samples and patient information were collected by XG, YY, NW and XL, data summary and statistical analysis were completed by MC and SZ. All authors read and approved the article and agreed to be accountable for all aspects of the research in ensuring that the accuracy or integrity of any part of the work was appropriately investigated and resolved.
Corresponding authors
Ethics declarations
Conflict of interest
There are no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cheng, M., Yang, F., Yang, Y. et al. Correlation analysis between camrelizumab trough concentration levels and efficacy or safety in East Asian patients with advanced lung cancer. Cancer Chemother Pharmacol 93, 31–39 (2024). https://doi.org/10.1007/s00280-023-04590-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-023-04590-z