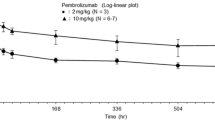
Abstract
Pembrolizumab is a monoclonal antibody that targets the programmed death-1 receptor to induce immune-mediated clearance (CL) of tumor cells. Originally approved by the US Food and Drug Administration in 2014 for treating patients with unresectable or metastatic melanoma, pembrolizumab is now also used to treat patients with non-small-cell lung cancer, classical Hodgkin lymphoma, head and neck cancer, and urothelial cancer. This paper describes the recently identified feature of pembrolizumab pharmacokinetics, the time-dependent or time-varying CL. Overall results indicate that CL decreases over the treatment period of a typical patient in a pattern well described by a sigmoidal function of time with three parameters: the maximum proportion change in CL from baseline (approximately Imax or exactly eImax − 1), the time to reach Imax/2 (TI50), and a Hill coefficient. Best overall response per response evaluation criteria in solid tumor category was found to be associated with the magnitude of Imax.






Similar content being viewed by others
References
Disis ML (2010) Immune regulation of cancer. J Clin Oncol 28:4531–4538
Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB et al (2002) Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med 8:793–800
Sharpe AH, Freeman GJ (2002) The B7-CD28 superfamily. Nat Rev Immunol 2:116–126
Brown JA, Dorfman DM, Ma F-R, Sullivan EL, Munoz O, Wood CR et al (2003) Blockade of programmed death-1 ligands on dendritic cells enhances T cell activation and cytokine production. J Immunol 170:1257–1266
Francisco LM, Sage PT, Sharpe AH (2010) The PD-1 pathway in tolerance and autoimmunity. Immunol Rev 236:219–242
Thompson RH, Dong H, Lohse CM, Leibovich BC, Blute ML, Cheville JC et al (2007) PD-1 expressed by tumor-infiltrating immune cells and is associated with poor outcome for patients with renal cell carcinoma. Clin Cancer Res 13:1757–1761
Nomi T, Sho M, Akahori T, Hamada K, Kubo A, Kanehiro H et al (2007) Clinical significance and therapeutic potential of the programmed death-1 ligand/programmed death-1 pathway in human pancreatic cancer. Clin Cancer Res 13:2151–2157
Gao Q, Wang X-Y, Qiu S-J, Yamato I, Sho M, Nakajima Y et al (2009) Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clin Cancer Res 15:971–979
Hamanishi J, Mandai M, Iwasaki M, Okazaki T, Tanaka Y, Yamaguchi K et al (2007) Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci USA 104:3360–3365
Mu C-Y, Huang J-A, Chen Y, Chen C, Zhang X-G (2011) High expression of PD-L1 in lung cancer may contribute to poor prognosis and tumor cells immune escape through suppressing tumor infiltrating dendritic cells maturation. Med Oncol 28:682–688
Fourcade J, Kudela P, Sun Z, Shen H, Land SR, Lenzner D et al (2009) PD-1 is a regulator of NY-ESO-1-specific CD8+ T cell expansion in melanoma patients. J Immunol 182:5240–5249
Blank C, Mackensen A (2007) Contribution of the PD-L1/PD-1 pathway to T-cell exhaustion: and update on implications for chronic infections and tumor evasion. Cancer Immunol Immunother 56:739–745
Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N (2002) Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci USA 99:12293–12297
Tsushima F, Tanaka K, Otsuki N, Youngnak P, Iwai H, Omura K et al (2006) Predominant expression of B7-H1 and its immunoregulatory roles in oral squamous cell carcinoma. Oral Oncol 42:268–274
Sznol M, Powderly JD, Smith DC, Brahmer JR, Drake CG, McDermott DF et al (2010) Safety and antitumor activity of biweekly MDX-1106 (anti-PD-1, BMS-936558/ONO-4538) in patients with advanced refractory malignancies [Abstract]. J Clin Oncol 28(15s):2506
Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, Daud A, Carlino MS, McNeil C, Lotem M, Larkin J, Lorigan P, Neyns B, Blank CU, Hamid O, Mateus C, Shapira-Frommer R, Kosh M, Zhou H, Ibrahim N, Ebbinghaus S, Ribas A, KEYNOTE-006 Investigators (2015) Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med 372(26):2521–2532
Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L, Carcereny E, Ahn MJ, Felip E, Lee JS, Hellmann MD, Hamid O, Goldman JW, Soria JC, Dolled-Filhart M, Rutledge RZ, Zhang J, Lunceford JK, Rangwala R, Lubiniecki GM, Roach C, Emancipator K, Gandhi L, KEYNOTE-001 Investigators (2015) Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 372(21):2018–2028
Herbst RS, Baas P, Kim DW, Felip E, Pérez-Gracia JL, Han JY, Molina J, Kim JH, Arvis CD, Ahn MJ, Majem M, Fidler MJ, de Castro G, Garrido M Jr, Lubiniecki GM, Shentu Y, Im E, Dolled-Filhart M, Garon EB (2016) Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 387(10027):1540–1550
Ribas A, Puzanov I, Dummer R, Schadendorf D, Hamid O, Robert C, Hodi FS, Schachter J, Pavlick AC, Lewis KD, Cranmer LD, Blank CU, O’Day SJ, Ascierto PA, Salama AK, Margolin KA, Loquai C, Eigentler TK, Gangadhar TC, Carlino MS, Agarwala SS, Moschos SJ, Sosman JA, Goldinger SM, Shapira-Frommer R, Gonzalez R, Kirkwood JM, Wolchok JD, Eggermont A, Li XN, Zhou W, Zernhelt AM, Lis J, Ebbinghaus S, Kang SP, Daud A (2015) Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol 16(8):908–918
Seiwert TY, Burtness B, Mehra R, Weiss J, Berger R, Eder JP, Heath K, McClanahan T, Lunceford J, Gause C, Cheng JD, Chow LQ (2016) Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): an open-label, multicentre, phase 1b trial. Lancet Oncol 17(7):956–965
Armand P, Shipp MA, Ribrag V, Michot JM, Zinzani PL, Kuruvilla J, Snyder ES, Ricart AD, Balakumaran A, Rose S, Moskowitz CH (2016) Programmed death-1 blockade with pembrolizumab in patients with classical hodgkin lymphoma after brentuximab vedotin failure. J Clin Oncol 34(31):3733–3739
Burki TK (2016) Pembrolizumab for classical Hodgkin’s lymphoma. Lancet Oncol 17(8):e324
Shoo BA, Kashani-Sabet M (2009) Melanoma arising in African-, Asian-, Latino- and Native-American populations. Semin Cutan Med Surg 28:96–102
Addeo R, Caraglia M, Iuliano G (2016) Pembrolizumab has antitumor activity in advanced head and neck cancer. Cancer Discov 6(7):693
de Greef R, Elassaiss-Schaap J, Chatterjee M et al (2017) Pembrolizumab: role of modeling and simulation in bringing a novel immunotherapy to patients with melanoma. CPT Pharmacomet Syst Pharmacol 6(1):5–7
Ahamadi M, Freshwater T, Prohn M et al (2017) Model-based characterization of the pharmacokinetics of pembrolizumab: a humanized anti-PD-1 monoclonal antibody in advanced solid tumors. CPT Pharmacomet Syst Pharmacol 6(1):49–57
Drug label for pembrolizumab BLA125514, Supplement 8. http://www.accessdata.fda.gov/drugsatfda_docs/label/2016/125514s008s012lbl.pdf. Accessed October 2016
Bergstrand M, Hooker AC, Wallin JE, Karlsson MO (2011) Prediction-corrected visual predictive checks for diagnosing nonlinear mixed-effects models. AAPS J 13:143–152
Keizer RJ, Karlsson MO, Hooker A (2013) Modeling and simulation workbench for NONMEM: tutorial on Pirana, PsN, and Xpose. CPT Pharmacomet Syst Pharmacol 2(e50):1–9
Chatterjee M, Turner DC, Felip E et al (2016) Systematic evaluation of pembrolizumab dosing in patients with advanced non-small-cell lung cancer. Ann Oncol 27(7):1291–1298
Fearon KC, Hansell DT, Preston T et al (1998) Influence of whole body protein turnover rate on resting energy expenditure in patients with cancer. Cancer Res 48:2590–2595
Roxburgh CS, McMillan DC (2014) Cancer and systemic inflammation: treat the tumour and treat the host. Br J Cancer 110:1409–1412
Perez Ruixo JJ, Doshi S, Sohn W, Chow A (2015) Quantitative pharmacology of denosumab in patients with bone metastases from solid tumors. J Clin Pharmacol 55(Suppl 3):S85–S92
Panoilia E, Schindler E, Samantas E, Aravantinos G, Kalofonos HP, Christodoulou C, Patrinos GP, Friberg LE, Sivolapenko G (2015) A pharmacokinetic binding model for bevacizumab and VEGF165 in colorectal cancer patients. Cancer Chemother Pharmacol 75:791–803
Thai HT, Veyrat-Follet C, Mentré F, Comets E (2013) Population pharmacokinetic analysis of free and bound aflibercept in patients with advanced solid tumors. Cancer Chemother Pharmacol 72:167–180
Bajaj G, Wang X, Agrawal S, Gupta M, Roy A, Feng Y (2017) Model-based population pharmacokinetic analysis of nivolumab in patients with solid tumors. CPT Pharmacomet Syst Pharmacol 6(1):58–66
Squibb BM (2011) Clinical pharmacology and biopharmaceutic review [of ipilimumab]. FDA Center for Drug Evaluation and Research (CDER), p 1–122
Yang J, Zhao H, Garnett C et al (2013) The combination of exposure–response and case–control analyses in regulatory decision making. J Clin Pharmacol 53:160–166
Liu J, Wang Y, Zhao L (2015) Assessment of exposure–response and case–control analyses in oncology using simulation based approach. In: 2015 American conference of pharmacometrics
Wang Y (2016) Special considerations for modeling exposure–response for biologics, 8 March 2016, San Diego, CA, USA. American Society for Clinical Pharmacology and Therapeutics (ASCPT)
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, H., Yu, J., Liu, C. et al. Time dependent pharmacokinetics of pembrolizumab in patients with solid tumor and its correlation with best overall response. J Pharmacokinet Pharmacodyn 44, 403–414 (2017). https://doi.org/10.1007/s10928-017-9528-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10928-017-9528-y