Abstract
Purpose
Ceramide is a sphingolipid metabolite that deactivates multiple oncogenic signaling pathways and promotes cell death. In-vivo data demonstrate single-agent anti-cancer activity and enhanced efficacy with combination strategies. This phase I dose-escalation trial evaluated Ceramide nanoLiposomes (CNL) in patients with advanced solid tumors and no standard treatment option.
Methods
The primary objective was to establish the maximum tolerated dose. Secondary objectives included determining the recommended phase II dose, the safety and tolerability, the pharmacokinetic profile and preliminary anti-tumor efficacy.
Results
15 patients with heavily pretreated metastatic disease enrolled. Safety data were analyzed for all patients, while pharmacokinetic data were available for 14 patients. There were no grade 3 or higher treatment-related adverse events. The maximum tolerated dose was not reached and there were no dose-limiting toxicities. The most common grade 1 or 2 treatment-related adverse events included headache, fatigue, constipation, nausea and transaminitis. The maximum concentration and area under the curve increased with dose. Clearance was consistent between doses and was observed mainly through the liver without significant hepatotoxicity. The half-life ranged from 20 to 30 h and the volume of distribution was consistent with a lipophilic drug.
Conclusions
CNL exhibited an encouraging safety profile and pharmacokinetic parameters, with some signals of efficacy including prolonged stable disease in 1 patient with refractory pancreatic cancer. Pre-clinical data indicate potential synergy between CNL and multiple systemic therapies including chemotherapy, targeted therapy, and immunotherapy. Future studies are planned investigating CNL in combination strategies.
Trial registration
This study is registered under ClinicalTrials.gov ID: NCT02834611.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dysfunctional sphingolipid metabolism is now being recognized as a hallmark of oncogenesis [1,2,3]. Ceramide, a sphingolipid metabolite is linked to preferential growth arrest and cell death of transformed cells. Mechanistically, ceramide facilitates the dephosphorylation of pro-mitogenic signaling cascades, including AKT, ERK and STAT3, in part through the activation of PKCζ and PP1/PP2A protein phosphatases [4,5,6,7]. Chemotherapeutic agents of diverse classes including gemcitabine, irinotecan, and etoposide lead to increased levels of endogenous ceramides and the addition of exogeneous ceramide augments the efficacy of multiple systemic therapy regimens [8, 9]. Relapsed or refractory disease is often associated with upregulation of enzymes that metabolize ceramide to less pro-apoptotic species, including sphingosine-1-phosphate, glycosphingolipids, or sphingomyelin [10]. Thus, restoring elevated endogenous levels of ceramide may be an effective strategy to limit cancer growth.
Multiple pre-clinical studies indicate that targeting sphingolipid metabolism by increasing ceramide levels or inhibiting sphingosine-1-phosphate-mediated cell growth might provide a novel avenue to overcome chemoresistance in advanced malignancies [2, 11]. The administration of exogenous short-chain ceramide (C6-ceramide) is an example of such an approach. Short-chain ceramides are selectively cytotoxic, promoting cell death in several neoplastic cell lines, including breast cancer, melanoma, hepatocellular carcinoma (HCC) and pancreatic cancer [12]. However, its clinical utility is limited by low solubility and poor cell permeability. Polyethylene-glycolated liposomes containing C6-ceramide were developed to overcome these pharmacologic limitations [13]. Ceramide nanoLiposomes (CNL) enhance ceramide’s potency against tumor cells in-vitro and demonstrate anti-cancer activity in animal models of HCC, pancreatic cancer, breast cancer, chronic lymphocytic leukemia, acute myeloid leukemia and natural killer large granulocytic leukemia [5, 10, 14,15,16,17,18]. In-vivo pharmacokinetic and toxicological studies in rats and dogs showed that CNL is well-tolerated [13]. Based on this promising pre-clinical safety and efficacy data, a phase I dose-escalation study was initiated to determine the maximum tolerated dose (MTD) of CNL in patients with advanced solid tumors. Secondary objectives were to determine the recommended phase II dose of CNL, its safety and tolerability, its pharmacokinetic profile and preliminary anti-tumor efficacy.
Materials and methods
Patient selection
The trial population included patients with a histologic or cytologic diagnosis of an advanced solid tumor without a curative or standard chemotherapy treatment option. Patients had to be ≥ 18 years of age, have an Eastern Cooperative Oncology Group performance status ≤ 2 and have a life expectancy ≥ 12 weeks. Eligibility required adequate hepatic, renal, and bone marrow function and a washout period of at least 4 weeks (6 weeks for mitoxantrone or mitomycin therapy). Patients were excluded if they had uncontrolled central nervous system (CNS) disease, a primary CNS malignancy, leptomeningeal disease, symptomatic heart failure, myocardial infarction within the prior 6 months, or a positive test for HIV, hepatitis B surface antigen or hepatitis C.
Treatment plan
This was a multi-center trial performed at the University of Maryland Greenebaum Comprehensive Cancer Center, the University of Virginia Emily Couric Cancer Center, and the Medical University of South Carolina Fred Hollings Cancer Center. This study was approved by the institutional review board at each participating site and conducted in accordance with the ethical standards established in the Declaration of Helsinki.
CNL was administered intravenously in a 250 mL bag of normal saline solution over approximately 2 h. It was given twice weekly on Mondays and Thursdays continuously until disease progression or unacceptable toxicity. One cycle was defined as 4 weeks of therapy. To minimize the risk of an infusion reaction observed with other lipid formulations, premedication with dexamethasone (20 mg), diphenhydramine (25 mg) and famotidine (20 mg) was given intravenously 30 min prior to each CNL infusion.
The study used an accelerated titration design to minimize patient exposure to subtherapeutic dose levels and allow rapid dose escalation. The initial dose level (dose level 1) was 36 mg/m2 and was determined from the MTD in canine studies. If there was no grade 2 toxicity suspected to be related to the investigational drug after 1 cycle, the next patient would enter at dose level 2. Upon the first instance of grade 2 toxicity of any type suspected to be related to the investigational drug, the cohort was to be expanded to use a traditional 3 + 3 design for that dose level. The MTD was defined as the dose level at which no more than 1 participant had a dose-limiting toxicity (DLT). This dose would then be the recommended phase II dose. Toxicities were graded according to the National Cancer Institute's Common Terminology Criteria for Adverse Events version 4.0.
Safety assessments
Clinical history, vital signs, and physical exam were performed weekly for the initial 8 weeks and then monthly. Complete blood counts and serum chemistries were performed weekly, and lipid profile and urinalysis were done monthly. Electrocardiogram was done at baseline, prior to the 1st and 2nd infusion and then every 4 weeks. Any hematologic or non-hematologic toxicity excluding alopecia that was ≥ grade 3 and suspected to be related to the investigational drug was considered a DLT. In addition, grade 3 nausea, vomiting, diarrhea or stomatitis of less than 5 day duration was not considered a DLT. A CNS toxicity ≥ grade 2 was considered a DLT.
Tumor response assessment
Anti-tumor activity was based on imaging and clinical assessments at baseline, every 2 cycles, and at the end of the study. Radiographic response assessment was based on Response Evaluation Criteria in Solid Tumors (RECIST version 1.1). Only those patients who had measurable disease present at baseline, received at least one cycle of therapy, and had their disease re-evaluated were considered evaluable for response.
Pharmacokinetics and metabolism
Pharmacokinetic (PK) samples were collected prior to the 1st, 2nd, 4th, and 8th infusions of Cycle 1. In addition, samples were taken at 30 min, 1 h, 1.5 h, 2 h, 3 h, 4 h, 6 h, 24 h, and 48 h after the 1st infusion and at 2 h and 24 h after the 2nd infusion. Maximum concentration (Cmax), half-life (T1/2) and clearance of CNL were determined based on PK analysis. AUC was determined using the linear trapezoidal method from 0 to 48 h (AUC0-48 h). The PK samples were evaluated by the University of Maryland’s School of Pharmacy for C6-ceramide content in plasma. Plasma was spiked with the internal standard C6-ceramide-13C2,D2 and subjected to base hydrolysis and organic extraction. Extracts were run on a Waters Acquity using a Phenomenex Kinetic HILIC 2.6 μm, 4.6 × 100 cm column with an isocratic elution using acetonitrile:water 50:50% v/v with 0.1% formic acid at 0.4mL/minute and 30 °C. Eluate was analyzed by an inline Water TQ-S mass spectrometer by multiple reaction monitoring. C6-ceramide levels were quantified with an external calibration curve and non-compartmental PK analyses performed using Phoenix WinNonlin software. C6 ceramide metabolism was evaluated by the University of Virginia Cancer Center’s Lipidomic and Metabolomic Shared Resource as described elsewhere [18].
Statistical analysis
The analytic data set included only patients who received any treatment concordant with the study protocol. The distribution of the DLT was estimated in the search for the MTD. All patients were evaluable for toxicities. Adverse events categorized as possibly, probably, or definitely related to treatment were assessed.
Results
Patients
15 eligible patients were enrolled on study. Baseline characteristics of all patients are outlined in Table 1. There were 7 male patients and 8 females. 80% were White and 20% were African-American. 1 patient identified as Hispanic/Latino. The ages of participants ranged from 44 to 83 years with a median age of 58. Patients in this study had metastatic disease and were heavily pre-treated with a median of 4 prior lines of systemic therapy. All patients had received prior surgery and 87% (13/15) had received prior radiation therapy.
DLTs, MTD and adverse event profile
Patients received CNL per the dosing schema outlined in Table 2. Dose level 1 started at 36 mg/m2 and was increased per protocol to 323 mg/m2. There were no DLT nor MTD identified.
Adverse events (AE) were determined in all patients who received at least 1 dose of study drug (Table 3). All patients experienced at least 1 treatment-emergent AE (TEAE) during the study, and 47% (7/15) experienced at least 1 grade ≥ 3 TEAE. Any-grade treatment-related AE (TRAE) were experienced by 53% (8/15). Importantly, there were no grade ≥ 3 TRAE. 20% (3/15) experienced a serious adverse event (SAE), but none were considered treatment-related. The most common clinical TRAE were headache in 20% (3/15) and constipation, nausea and fatigue each present in 13% (2/15) of patients. Other clinical TRAE present in 7% (1/15) included chills, diarrhea, facial edema, distal neuropathy, abdominal pain, bone pain and facial flushing. The most common laboratory TRAE were increased aspartate aminotransferase (AST) in 20% (3/15), and increased alanine aminotransferase (ALT) and alkaline phosphatase (AlkPhos) each in 13% (2/15). Other laboratory TRAE present in 7% (1/15) included thrombocytopenia, hypoalbuminemia and hypocalcemia. All TRAE were either grade 1 or 2 in severity and none led to dose reduction or discontinuation of the study drug.
Pharmacokinetics
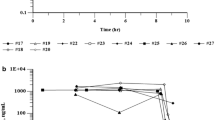
PK data was available and analyzed for 14 patients and is outlined in Table 4. Cmax and area under the concentration–time curve (AUC) both increased with dose with r2 for Cmax of 0.9126 and for AUC of 0.8393. The small number of patients in each cohort might have contributed to a degree of variability in the dose linear relationship. Clearance and volume of distribution were consistent between doses. Pre-clinical data indicate that C6-ceramide is preferentially cleared by the liver [13, 19] T1/2 ranged from 20 to 30 h and the steady state volume of distribution (Vss) was consistent with a lipophilic drug. The ceramide plasma concentration, which correlates with pre-clinical efficacious dose levels of 36 mg/kg, using allometric scaling, is 108 mg/m2 [13, 20]. Importantly, ceramide concentrations above this threshold were achieved without DLT. Targeted LC/MS/MS revealed that a portion of exogenous C6-ceramide was metabolized into C6-sphingomyelin (SM), C6-hexosylceramide (HexCer), and C6-dihexosylceramide (diHexCer) (Fig. 1B–D). C6-ceramide-1-phosphate was not detected. Of note, PK samples for the subject who received 323 mg/m2 were lost during shipping.
Anti-tumor activity
13 patients were evaluable for response to CNL (Table 5). These patients represented a range of tumor types with refractory metastatic disease. Patients received a median of 15 doses of CNL. No complete or partial responses were observed. 38% (5/13) of participants demonstrated stable disease at 8 weeks and 1 patient with pancreatic cancer showed disease stability for greater than 4 months.
Discussion
This phase I study of CNL in patients with advanced and refractory solid tumors demonstrated reasonable safety and tolerability with no clinically significant TRAE. No DLT was observed and a MTD was not reached. The human data in this study is consistent with our pre-clinical animal data including a long T1/2, large AUC and volume of distribution with minimal toxicity [13]. Of note, the AUC and Cmax increased with CNL dose without adverse effects. The trial was stopped at a dose of 323 mg/m2 due to allowable deliverable volumes. While steady state volume of distribution values were consistent with a lipophilic drug formulation, leaner patients appeared more likely to respond with stable disease compared to those with a higher body mass index. This may reflect distribution and metabolism within adipose tissues and may require further evaluation to determine if dosing regimens should be altered based on body habitus. Pre-clinical data in a rat model using CNL show extensive tissue distribution and indicate that preferential clearance is by the liver [19]. We now also demonstrate that metabolism of C6-ceramide to other C6-metabolites can be detected in the circulation, which may be attributed to metabolizing enzymes in the bloodstream, liver or through interaction with hematopoietic cells. The levels of C6-SM and C6-HexCer appear to peak with C6-ceramide, whereas generation of the higher ordered glycosphingolipid, C6-diHexCer, may be slower to generate (see Fig. 1). Future clinical studies may determine if these profiles reflect changes in efficacy. The twice weekly dosing was determined from pre-clinical experiments in rodents and beagles to achieve steady state levels, where the T1/2 was calculated at approximately 20 h. Of note, based upon the lack of significant adverse events, the FDA allowed transition from a 3 + 3 strategy back to 1 patient per dose at 122 mg/m2, which reduced the time to study completion, but also limited the total number of patients enrolled.
CNL was developed to synergize with standard-of-care (SOC) approaches based on strong pre-clinical combinatorial activity [10, 21, 22]. CNL can reduce pro-survival gene products such as survivin or MCL-1, induced by standard chemotherapeutic and targeted agents [6, 23]. Moreover, numerous SOC therapeutics can induce enzymatic cascades to increase ceramide formation [8]. Recent in-vivo data also supports an immunomodulatory role for CNL in counteracting the suppressive microenvironment and possible synergy with checkpoint blockade [24]. Thus, it was encouraging to see some degree of efficacy as monotherapy, with 38% of patients demonstrating stable disease in 4 distinct cancer subtypes. The fact that no clinically significant toxicities were noted across 10 different cancers also bodes well for continued evaluation in combination strategies. As a MTD was not reached and efficacy signals were seen beginning at 81 mg/m2, we envision a phase II adaptive trial design where CNL is evaluated at 81 mg/m2 or 121 mg/m2 combined with immunotherapy or cytotoxic chemotherapy. Due to potential synergy, we may be able to use lower doses of standard therapeutics to obtain desired effects or resurrect previous lines of therapy in patients who have become resistant to SOC.
While there are several nanoliposomes or nanoparticles developed as therapeutics, including Doxil (Doxorubicin), DaunoXome (Daunorubicin), Depoct (Cytarabine), Marquibo (Vinblastine), Onivyde (Irinotecan) and Abraxane (Paclitaxel), there are no nanoscale drug delivery systems for bioactive lipids such as ceramides. While the preclinical development of CNL has been described, there are certain distinct engineered parameters that allow for an improved pharmacokinetic and pharmacodynamic profile [13]. For example, CNL is designed to deliver bioactive ceramide via inter-bilayer movement instead of membrane fusion or active receptor targeting, which directs ceramide to the plasmalemmae instead of exosome/lysosome processing [19]. In addition, the dual pegylation strategy used to manufacture CNL assures both long-term shelf-life (2 years) and biological stability [13]. Finally, all patients were pre-treated with anti-histamines and steroids as a precaution, since many nanoliposomes can induce an anaphylactic reaction, and both CNL and the ghost liposomes induced transient local erythema and edema and changes in heart rate and blood pressure readings in beagle dogs [13]. Importantly, with administration of pre-medication, no evidence of hypersensitivity including anaphylaxis was seen for patients on this study.
In sum, CNL exhibited an encouraging safety profile, as well as a long T1/2, and large AUC and volume of distribution with some signals of efficacy including prolonged stable disease in 1 patient with pancreatic cancer. These phase I results along with strong pre-clinical data indicating synergy with systemic agents including chemotherapy, targeted therapy and immunotherapy support further investigation of CNL in combination strategies.
Data availability
The data sets generated during the current study and subsequently analyzed are available from the corresponding author on reasonable request.
References
Hannun YA, Obeid LM (2018) Sphingolipids and their metabolism in physiology and disease. Nat Rev Mol Cell Biol 19(3):175–191
Ogretmen B (2018) Sphingolipid metabolism in cancer signalling and therapy. Nat Rev Cancer 18(1):33–50
Ung J et al (2022) Harnessing the power of sphingolipids: prospects for acute myeloid leukemia. Blood Rev 55:100950
Bourbon NA, Sandirasegarane L, Kester M (2002) Ceramide-induced inhibition of Akt is mediated through protein kinase Czeta: implications for growth arrest. J Biol Chem 277(5):3286–3292
Li G et al (2018) Nanoliposome C6-ceramide increases the anti-tumor immune response and slows growth of liver tumors in mice. Gastroenterology 154(4):1024-1036 e9
Doshi UA et al (2017) STAT3 mediates C6-ceramide-induced cell death in chronic lymphocytic leukemia. Sig Transduct Target Ther 2:17051
Fox TE et al (2007) Ceramide recruits and activates protein kinase C zeta (PKC zeta) within structured membrane microdomains. J Biol Chem 282(17):12450–12457
Shaw J et al (2018) Novel sphingolipid-based cancer therapeutics in the personalized medicine era. Adv Cancer Res 140:327–366
Morad SA, Cabot MC (2013) Ceramide-orchestrated signalling in cancer cells. Nat Rev Cancer 13(1):51–65
Barth BM et al (2019) Sphingolipid metabolism determines the therapeutic efficacy of nanoliposomal ceramide in acute myeloid leukemia. Blood Adv 3(17):2598–2603
Green CD et al (2021) Sphingolipids in metabolic disease: the good, the bad, and the unknown. Cell Metab 33(7):1293–1306
Barth BM, Cabot MC, Kester M (2011) Ceramide-based therapeutics for the treatment of cancer. Anticancer Agents Med Chem 11(9):911–919
Kester M et al (2015) Preclinical development of a C6-ceramide NanoLiposome, a novel sphingolipid therapeutic. Biol Chem 396(6–7):737–747
Jiang Y et al (2011) Combinatorial therapies improve the therapeutic efficacy of nanoliposomal ceramide for pancreatic cancer. Cancer Biol Ther 12(7):574–585
Stover TC et al (2005) Systemic delivery of liposomal short-chain ceramide limits solid tumor growth in murine models of breast adenocarcinoma. Clin Cancer Res 11(9):3465–3474
Ryland LK et al (2013) C6-ceramide nanoliposomes target the Warburg effect in chronic lymphocytic leukemia. PLoS One 8(12):e84648
Liu X et al (2010) Targeting of survivin by nanoliposomal ceramide induces complete remission in a rat model of NK-LGL leukemia. Blood 116(20):4192–4201
Khokhlatchev AV et al (2022) Ceramide nanoliposomes augment the efficacy of venetoclax and cytarabine in models of acute myeloid leukemia. FASEB J 36(10):e22514
Zolnik BS et al (2008) Rapid distribution of liposomal short-chain ceramide in vitro and in vivo. Drug Metab Dispos 36(8):1709–1715
Nair AB, Jacob S (2016) A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm 7(2):27–31
Adiseshaiah PP et al (2013) Synergistic combination therapy with nanoliposomal C6-ceramide and vinblastine is associated with autophagy dysfunction in hepatocarcinoma and colorectal cancer models. Cancer Lett 337(2):254–265
Tran MA et al (2008) Combining nanoliposomal ceramide with sorafenib synergistically inhibits melanoma and breast cancer cell survival to decrease tumor development. Clin Cancer Res 14(11):3571–3581
Pearson JM et al (2020) Ceramide analogue SACLAC modulates sphingolipid levels and MCL-1 splicing to induce apoptosis in acute myeloid leukemia. Mol Cancer Res 18(3):352–363
Qi X et al (2022) Nanoliposome C6-Ceramide in combination with anti-CTLA4 antibody improves anti-tumor immunity in hepatocellular cancer. FASEB J 36(4):e22250
Acknowledgements
We would like to acknowledge the contributions and efforts of Mark Kester, Robert Dreicer and Ed Sausville for the work included in this manuscript. This work was additionally supported by Department of Defense PC210475, National Cancer Institute Awards CA1719835 and CA208396, a Cancer Center Support Grant CA044579, and a National Cancer Institute Phase II Grant R44CA195793.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Penn State Research Foundation has licensed Ceramide nanoLiposomes (CNL) to Keystone Nano (KN) Inc. KN, who holds the IND for the phase I clinical trial, is developing CNL as Ceraxa™. Jeff Davidson is Chief Operating Officer of KN and Mylisa Parette previously served as Vice President of Research at KN. There are no other financial or non-financial conflicts of interest directly or indirectly related to this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ciner, A., Gourdin, T., Davidson, J. et al. A phase I study of the ceramide nanoliposome in patients with advanced solid tumors. Cancer Chemother Pharmacol 93, 23–29 (2024). https://doi.org/10.1007/s00280-023-04588-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-023-04588-7