Abstract
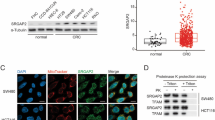
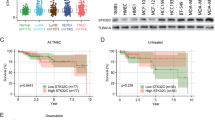
Doxorubicin-based chemotherapy remains as a major therapeutic approach for patients with triple-negative breast cancer (TNBC). However, insensitivity or resistance to doxorubicin treatment limits the therapeutic efficacy. Mitochondrial respiration plays a critical role in regulating the sensitivity of cancer cells to chemotherapy drugs. Here, we found that small trans-membrane and glycosylated protein (SMAGP) is upregulated in TNBC cells in comparison to normal breast and other subtypes of breast cancer cells. High SMAGP expression is associated with poorer overall survival of TNBC patients. Importantly, loss of SMAGP enhanced the sensitivity of TNBC cells to doxorubicin treatment. Mechanistically, we detected a functional pool of SMAGP in the mitochondria of TNBC cells controlling doxorubicin sensitivity via regulating mitochondrial respiration. Thus, our data suggest that SMAGP acts as a novel regulator of doxorubicin sensitivity in TNBC, identifying SMAGP as a promising therapeutic target for improving the efficacy of doxorubicin-based chemotherapy in TNBC patients.





Similar content being viewed by others
Data availability
Publicly available dataset analyzed in this study can be found here: TCGA (https://portal.gdc.cancer.gov/).
References
Siegel RL, Miller KD, Fuchs HE, Jemal A (2022) Cancer statistics. CA Cancer J Clin 72:7–33. https://doi.org/10.3322/caac.21708
Carey LA et al (2006) Race, breast cancer subtypes, and survival in the Carolina breast cancer study. JAMA 295:2492–2502. https://doi.org/10.1001/jama.295.21.2492
O’Brien KM et al (2010) Intrinsic breast tumor subtypes, race, and long-term survival in the Carolina breast cancer study. Clin Cancer Res 16:6100–6110. https://doi.org/10.1158/1078-0432.CCR-10-1533
Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V (2007) Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California cancer registry. Cancer 109:1721–1728. https://doi.org/10.1002/cncr.22618
Dent R et al (2007) Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res 13:4429–4434. https://doi.org/10.1158/1078-0432.ccr-06-3045
Ward PS, Thompson CB (2012) Metabolic reprogramming: a cancer hallmark even warburg did not anticipate. Cancer Cell 21:297–308. https://doi.org/10.1016/j.ccr.2012.02.014
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144:646–674. https://doi.org/10.1016/j.cell.2011.02.013
Lee KM et al (2017) MYC and MCL1 cooperatively promote chemotherapy-resistant breast cancer stem cells via regulation of mitochondrial oxidative phosphorylation. Cell Metab 26:633-647.e637. https://doi.org/10.1016/j.cmet.2017.09.009
Liang XJ et al (2008) SIRT1 contributes in part to cisplatin resistance in cancer cells by altering mitochondrial metabolism. Mol Cancer Res 6:1499–1506. https://doi.org/10.1158/1541-7786.MCR-07-2130
Tang Y, Liu G, Jia Y, Sun T (2022) SRGAP2 controls colorectal cancer chemosensitivity via regulation of mitochondrial complex I activity. Hum Cell. https://doi.org/10.1007/s13577-022-00781-7
Vellinga TT et al (2015) SIRT1/PGC1alpha-dependent increase in oxidative phosphorylation supports chemotherapy resistance of colon cancer. Clin Cancer Res 21:2870–2879. https://doi.org/10.1158/1078-0432.CCR-14-2290
Han XJ et al (2015) Mitochondrial dynamics regulates hypoxia-induced migration and antineoplastic activity of cisplatin in breast cancer cells. Int J Oncol 46:691–700. https://doi.org/10.3892/ijo.2014.2781
Sarmiento-Salinas FL et al (2019) Breast cancer subtypes present a differential production of reactive oxygen species (ros) and susceptibility to antioxidant treatment. Front Oncol 9:480. https://doi.org/10.3389/fonc.2019.00480
Zhao J et al (2013) Mitochondrial dynamics regulates migration and invasion of breast cancer cells. Oncogene 32:4814–4824. https://doi.org/10.1038/onc.2012.494
Park JH et al (2016) Fatty acid oxidation-driven src links mitochondrial energy reprogramming and oncogenic properties in triple-negative breast cancer. Cell Rep 14:2154–2165. https://doi.org/10.1016/j.celrep.2016.02.004
Jia Y, Li H, Liu G, Song F (2018) SMAGP a novel biomarker of cervical cancer development and progression. Onco Targets Ther 11:6925–6935. https://doi.org/10.2147/ott.s175808
Ni H, Ji D, Huang Z, Li J (2020) SMAGP knockdown inhibits the malignant phenotypes of glioblastoma cells by inactivating the PI3K/Akt pathway. Arch Biochem Biophys 695:108628. https://doi.org/10.1016/j.abb.2020.108628
Tarbé NG, Rio MC, Hummel S, Weidle UH, Zöller M (2005) Overexpression of the small transmembrane and glycosylated protein SMAGP supports metastasis formation of a rat pancreatic adenocarcinoma line. Int J Cancer 117:913–922. https://doi.org/10.1002/ijc.21275
Tarbé NG, Rio MC, Weidle UH (2004) SMAGP, a new small trans-membrane glycoprotein altered in cancer. Oncogene 23:3395–3403. https://doi.org/10.1038/sj.onc.1207469
Thul PJ et al (2017) A subcellular map of the human proteome. Science. https://doi.org/10.1126/science.aal3321
Li N et al (2003) Mitochondrial complex I inhibitor rotenone induces apoptosis through enhancing mitochondrial reactive oxygen species production. J Biol Chem 278:8516–8525. https://doi.org/10.1074/jbc.M210432200
Watabe M, Nakaki T (2007) Mitochondrial complex I inhibitor rotenone-elicited dopamine redistribution from vesicles to cytosol in human dopaminergic SH-SY5Y cells. J Pharmacol Exp Ther 323:499–507. https://doi.org/10.1124/jpet.107.127597
Eiermann N, Stoecklin G, Jovanovic B (2022) Mitochondrial inhibition by sodium azide induces assembly of eIF2α phosphorylation-independent stress granules in mammalian cells. Int J Mol Sci. https://doi.org/10.3390/ijms23105600
Ishii H, Shirai T, Makino C, Nishikata T (2014) Mitochondrial inhibitor sodium azide inhibits the reorganization of mitochondria-rich cytoplasm and the establishment of the anteroposterior axis in ascidian embryo. Dev Growth Differ 56:175–188. https://doi.org/10.1111/dgd.12117
Therneau, T. M. A Package for Survival Analysis in R. R package version 3.2–13. https://cran.r-project.org/package=survival (2021)
Therneau TM, Grambsch PM (2000) Modeling survival data: extending the Cox model. Springer, New York
Acknowledgements
We thank the TCGA Research Network for providing its platforms and valuable datasets.
Author information
Authors and Affiliations
Contributions
Conceptualization: HX and SH; resources: HX and SH; data curation: SH; formal analysis: HX, XY, and SH; supervision: SH; validation: HX, XY, and SH; investigation: HX and XY; visualization: HX and XY; methodology: HX and XY; project administration: SH; writing—original draft: HX; writing—review and editing: HX, XY, and SH.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
280_2022_4496_MOESM1_ESM.tif
Figure S1. Knockdown efficiencies of SMAGP-targeting shRNAs were determined in TNBC cells.Western blot analysis of SMAGP protein level in the indicated breast cancer cells transduced with shCtrl or two different shRNAs targeting SMAGP. (TIF 2129 kb)
280_2022_4496_MOESM2_ESM.tif
Figure S2. SMAGP depletion does not influence the cell viability, migration, or invasion of breast cancer cells. (A) Cell viabilities of various breast cancer cells transduced with shCtrl or two different SMAGP-targeting shRNAs were determined using SRB assay. (B and C) Boyden chamber-based migration (B) and invasion (C) assay were performed to determine the migratory and invasive capacities of various breast cancer cells upon transduction with shCtrl or two different shRNAs targeting SMAGP. (A-C) Data are presented as mean + SD. n = 3. Results are representative of a minimum of three independent experiments. (TIF 2327 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xiao, H., Yang, X. & Huang, S. SMAGP regulates doxorubicin sensitivity in triple-negative breast cancer cells via modulating mitochondrial respiration. Cancer Chemother Pharmacol 91, 43–52 (2023). https://doi.org/10.1007/s00280-022-04496-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-022-04496-2