Abstract
Purpose
Erlotinib is an essential drug for non-small cell lung cancer (NSCLC) patients harboring epidermal growth factor receptor (EGFR) activating mutations. The relationship between the pharmacokinetics and skin rash and diarrhea of erlotinib in Chinese patients with EGFR mutated NSCLC is unknown. In this study, we evaluated the variability in erlotinib trough concentration and its relationship with the severity of skin rash and diarrhea in patients with two common types of EGFR mutations: a deletion in exon 19 and point mutations in exon 21 L858R.
Patients and methods
EGFR mutation-positive Chinese patients (n = 52) treated with erlotinib were included in our study; the steady-state trough concentrations were assessed; and the occurrence and severity of skin rash and diarrhea after the onset of treatment with erlotinib were recorded. The patients were divided into two groups by mutation type (exon 19 deletions or exon 21 L858R point mutations). Occurrence and severity of skin rash and diarrhea was analyzed in both groups.
Results
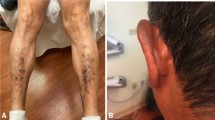
The overall mean (± SD) steady-state trough concentration for erlotinib was 1380 ± 663 ng/mL, and there was no significant difference of erlotinib concentrations between the two mutation groups. Occurrence and severity of skin rash was significantly associated with trough concentration in patients with exon 19 deletions but not exon 21 L858R point mutations. Significant association of erlotinib concentrations with diarrhea was found neither in the exon 19 deletions group nor in the exon 21 L858R point mutation group.
Conclusions
The occurrence and severity of skin rash correlated with increase in erlotinib trough concentrations only in Chinese patients with exon 19 deletion; the erlotinib trough concentrations were not associated with diarrhea.


Similar content being viewed by others
References
Xie CJJ, Bao X, Zhan W, Han T, Gan M, Zhang C, Wang J (2016) Inhibition of mitochondrial glutaminase activity reverses acquired erlotinib resistance in non-small cell lung cancer. Oncotarget 7(1):11
Maemondo MIA, Kobayashi K, Sugawara S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I, Fujita Y, Okinaga S, Hirano H, Yoshimori K, Harada T, Ogura T, Ando M, Miyazawa H, Tanaka T, Saijo Y, Hagiwara K, Morita S. Nukiwa T (2010) Gefitinib or chemotherapy for non-small cell lung cancer with mutated EGFR. N Engl J Med 362(25):8
Jamal-Hanjani M, Spicer J (2012) Epidermal growth factor receptor tyrosine kinase inhibitors in the treatment of epidermal growth factor receptor-mutant non-small cell lung cancer metastatic to the brain. Clin Cancer Res 18(4):938–944. https://doi.org/10.1158/1078-0432.CCR-11-2529
Shames DSW II (2014) The evolving genomic classification of lung cancer. J Pathol 232(2):121–133. https://doi.org/10.1002/path.4275
Hamada ASJ, Saeki S, Iwamoto N, Inaba M, Ushijima S, Urata M, Kishi H, Fujii S, Semba H, Kashiwabara K, Tsubata Y, Kai Y, Isobe T, Kohrogi H, Saito H (2012) Association of ABCB1 polymorphisms with erlotinib pharmacokinetics and toxicity in Japanese patients with non-small-cell lung cancer. Pharmacogenomics 13(5):9
Johnson BE, Janne PA (2005) Epidermal growth factor receptor mutations in patients with non-small cell lung cancer. Cancer Res 65(17):7525–7529. https://doi.org/10.1158/0008-5472.CAN-05-1257
Tournier N, Goutal S, Auvity S, Traxl A, Mairinger S, Wanek T, Helal OB, Buvat I, Soussan M, Caille F, Langer O (2017) Strategies to inhibit ABCB1- and ABCG2-mediated efflux transport of erlotinib at the blood-brain barrier: a PET study on nonhuman primates. J Nucl Med 58(1):117–122. https://doi.org/10.2967/jnumed.116.178665
Dempke WC, Edvardsen M, Lu K, Reinmuth S, Reck N, Inoue M A (2015) Brain metastases in NSCLC—are TKIs changing the treatment strategy? Anticancer Res 35(11):10
Arora A, Scholar EM (2005) Role of tyrosine kinase inhibitors in cancer therapy. J Pharmacol Exp Ther 315(3):971–979. https://doi.org/10.1124/jpet.105.084145
Shepherd FARPJ, Ciuleanu T, Tan EH. Hirsh V, Thongprasert S, Campos D, Maoleekoonpiroj S, Smylie M, Martins R, van Kooten M, Dediu M, Findlay B, Tu D, Johnston D, Bezjak A, Clark G, Santabárbara P, Seymour L (2005) Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med 353 (2):9
Ling J, Johnson KA, Miao Z, Rakhit A, Pantze MP, Hamilton M, Lum BL, Prakash C (2006) Metabolism and excretion of erlotinib, a small molecule inhibitor of epidermal growth factor receptor tyrosine kinase, in healthy male volunteers. Drug Metab Dispos Biol Fate Chem 34(3):420–426. https://doi.org/10.1124/dmd.105.007765
Shi Z, Peng XX, Kim IW, Shukla S, Si QS, Robey RW, Bates SE, Shen T, Ashby CR Jr, Fu LW, Ambudkar SV, Chen ZS (2007) Erlotinib (Tarceva, OSI-774) antagonizes ATP-binding cassette subfamily B member 1 and ATP-binding cassette subfamily G member 2-mediated drug resistance. Cancer Res 67(22):11012–11020. https://doi.org/10.1158/0008-5472.CAN-07-2686
Terada T, Noda S, Inui K (2015) Management of dose variability and side effects for individualized cancer pharmacotherapy with tyrosine kinase inhibitors. Pharmacol Ther 152:125–134. https://doi.org/10.1016/j.pharmthera.2015.05.009
Mitsudomi TMS, Yatabe Y, Negoro S, Okamoto I, Tsurutani J, Seto T, Satouchi M, Tada H, Hirashima T, Asami K, Katakami N, Takada M, Yoshioka H, Shibata K, Kudoh S, Shimizu E, Saito H, Toyooka S, Nakagawa K, Fukuoka M (2010) Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 11(2):8. https://doi.org/10.1016/s14702045(09)70364-x
Petrelli F, Borgonovo K, Barni S (2016) Preventing or treating anti-EGFR related skin rash with antibiotics? Ann Transl Med 4(16):312. https://doi.org/10.21037/atm.2016.07.01
Steffens M, Paul T, Hichert V, Scholl C, von Mallek D, Stelzer C, Sorgel F, Reiser B, Schumann C, Rudiger S, Boeck S, Heinemann V, Kachele V, Seufferlein T, Stingl J (2016) Dosing to rash?—The role of erlotinib metabolic ratio from patient serum in the search of predictive biomarkers for EGFR inhibitor-mediated skin rash. Eur J Cancer 55:131–139. https://doi.org/10.1016/j.ejca.2015.11.022
Zhou C, Wu Y-L, Chen G, Feng J, Liu X-Q, Wang C, Zhang S, Wang J, Zhou S, Ren S, Lu S, Zhang L, Hu C, Hu C, Luo Y, Chen L, Ye M, Huang J, Zhi X, Zhang Y, Xiu Q, Ma J, Zhang L, You C (2011) Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 12(8):735–742. https://doi.org/10.1016/s1470-2045(11)70184-x
Gao B, Yeap S, Clements A, Balakrishnar B, Wong M, Gurney H (2012) Evidence for therapeutic drug monitoring of targeted anticancer therapies. J Clin Oncol 30(32):4017–4025. https://doi.org/10.1200/JCO.2012.43.5362
Widmer N, Bardin C, Chatelut E, Paci A, Beijnen J, Leveque D, Veal G, Astier A (2014) Review of therapeutic drug monitoring of anticancer drugs part two-targeted therapies. Eur J Cancer 50(12):2020–2036. https://doi.org/10.1016/j.ejca.2014.04.015
Klumpen HJ, Samer CF, Mathijssen RH, Schellens JH, Gurney H (2011) Moving towards dose individualization of tyrosine kinase inhibitors. Cancer Treat Rev 37(4):251–260. https://doi.org/10.1016/j.ctrv.2010.08.006
Lu JF, Eppler SM, Wolf J, Hamilton M, Rakhit A, Bruno R, Lum BL (2006) Clinical pharmacokinetics of erlotinib in patients with solid tumors and exposure-safety relationship in patients with non-small cell lung cancer. Clin Pharm Ther 80(2):136–145. https://doi.org/10.1016/j.clpt.2006.04.007
White-Koning M, Civade E, Geoerger B, Thomas F, Le Deley MC, Hennebelle I, Delord JP, Chatelut E, Vassal G (2011) Population analysis of erlotinib in adults and children reveals pharmacokinetic characteristics as the main factor explaining tolerance particularities in children. Clin Cancer Res 17(14):4862–4871. https://doi.org/10.1158/1078-0432.CCR-10-3278
Basch E, Iasonos A, McDonough T, Barz A, Culkin A, Kris MG, Scher HI, Schrag D (2006) Patient versus clinician symptom reporting using the National Cancer Institute Common Terminology Criteria for Adverse Events: results of a questionnaire-based study. Lancet Oncol 7(11):903–909. https://doi.org/10.1016/s1470-2045(06)70910-x
Hidalgo MSLL, Nemunaitis J, Rizzo J, Hammond LA, Takimoto C, Eckhardt SG, Tolcher A, Britten CD, Denis L, Ferrante K, Von Hoff DD, Silberman S, Rowinsky EK (2001) Phase I and pharmacologic study of OSI-774, an epidermal growth factor receptor tyrosine kinase inhibitor, in patients with advanced solid malignancies. J Clin Oncol 19(13):12
Petit-Jean EBT, Guidi M, Quoix E, Gourieux B, Decosterd LA, Gairard-Dory A-C, Ubeaud-Séquier G, Widmer N (2015) Erlotinib: another candidate for the therapeutic drug monitoring of targeted therapy of cancer? A pharmacokinetic and pharmacodynamic systematic review of literature. Ther Drug Monit 37(1):19
Druker BJTM, Resta DJ, Peng B, Buchdunger E, Ford JM, Lydon NB, Kantarjian H, Capdeville R, Ohno-Jones S, Sawyers CL (2001) Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med 344(14):7
Hamilton M, Wolf JL, Rusk J, Beard SE, Clark GM, Witt K, Cagnoni PJ (2006) Effects of smoking on the pharmacokinetics of erlotinib. Clin Cancer Res 12(7 Pt 1):2166–2171. https://doi.org/10.1158/1078-0432.CCR-05-2235
Segaert SCG, Lemmens L, Dumon K, Van CE, Tejpar S (2009) Skin toxicities of targeted therapies. Eur J Cancer 45(S1):13
Hu JC, Sadeghi P, Pinter-Brown LC, Yashar S, Chiu MW (2007) Cutaneous side effects of epidermal growth factor receptor inhibitors: clinical presentation, pathogenesis, and management. J Am Acad Dermatol 56(2):317–326. https://doi.org/10.1016/j.jaad.2006.09.005
Fiala O, Hosek P, Pesek M, Finek J, Racek J, Stehlik P, Sorejs O, Minarik M, Benesova L, Celer A, Nemcova I, Kucera R, Topolcan O (2017) Serum concentration of erlotinib and its correlation with outcome and toxicity in patients with advanced-stage NSCLC. Anticancer Res 37(11):6469–6476. https://doi.org/10.21873/anticanres.12102
Hichert VSC, Steffens M, Paul T, Schumann C, Rüdiger S, Boeck S, Heinemann V, Kächele V, Seufferlein T, Stingl J (2017) Predictive blood plasma biomarkers for EGFR inhibitor-induced skin rash. Oncotarget 8(21):11
Wei F, Shin D, Cai X (2017) Incidence, risk and prognostic role of anti-epidermal growth factor receptor-induced skin rash in biliary cancer: a meta-analysis. Int J Clin Oncol. https://doi.org/10.1007/s10147-017-1231-x
Okabe T, Okamoto I, Tamura K, Terashima M, Yoshida T, Satoh T, Takada M, Fukuoka M, Nakagawa K (2007) Differential constitutive activation of the epidermal growth factor receptor in non-small cell lung cancer cells bearing EGFR gene mutation and amplification. Cancer Res 67(5):2046–2053. https://doi.org/10.1158/0008-5472.CAN-06-3339
Okuda Y, Sato K, Sudo K, Hasegawa Y, Asano M, Miura H, Takeda M, Sano M, Watanabe H, Kobayashi H, Niioka T, Miura M, Ito H (2017) Low plasma concentration of gefitinib in patients with EGFR exon 21 L858R point mutations shortens progression-free survival. Cancer Chemother Pharmacol 79(5):1013–1020. https://doi.org/10.1007/s00280-017-3285-x
Zhu JQ, Zhong WZ, Zhang GC, Li R, Zhang XC, Guo AL, Zhang YF, An SJ, Mok TS, Wu YL (2008) Better survival with EGFR exon 19 than exon 21 mutations in gefitinib-treated non-small cell lung cancer patients is due to differential inhibition of downstream signals. Cancer Lett 265(2):307–317. https://doi.org/10.1016/j.canlet.2008.02.064
Jaka A, Gutierrez-Rivera A, Ormaechea N, Blanco J, La Casta A, Sarasqueta C, Izeta A, Tuneu A (2014) Association between EGFR gene polymorphisms, skin rash and response to anti-EGFR therapy in metastatic colorectal cancer patients. Exp Dermatol 23(10):751–753. https://doi.org/10.1111/exd.12510
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant number 81603206), Hunan Provincial Pharmaceutical Association Fund (Grant number hn2017005), and Health and Family Planning Commission Foundation of Hunan Province (Grant number B20180252).
Author information
Authors and Affiliations
Contributions
DL, NY, and DX designed the study and wrote the protocol. DL, NL, YZ, WJ, and CZ performed the experiments, analyzed the data, and revised the manuscript. DY and LC contributed to the reagents and materials. DL drafted the manuscript. DL and NY revised the manuscript content. All the authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Liao, D., Yao, D., Liu, N. et al. Correlation of plasma erlotinib trough concentration with skin rash in Chinese NSCLC patients harboring exon 19 deletion mutation. Cancer Chemother Pharmacol 82, 551–559 (2018). https://doi.org/10.1007/s00280-018-3642-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-018-3642-4