Abstract
Purpose
To inform lumretuzumab and pertuzumab dose modifications in order to decrease the incidence, severity, and duration of the diarrhea events in metastatic breast cancer patients treated with a combination therapy of lumretuzumab (anti-HER3) in combination with pertuzumab (anti-HER2) and paclitaxel using quantitative clinical pharmacology modeling approaches.
Methods
The safety and pharmacokinetic (PK) data from three clinical trials (lumretuzumab monotherapy n = 47, pertuzumab monotherapy n = 78, and the combination therapy of lumretuzumab, pertuzumab and paclitaxel n = 35) were pooled together to develop a continuous-time discrete states Markov model describing the dynamics of the diarrhea events.
Results
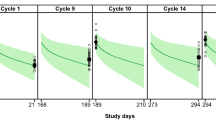
The model was able to capture the time course of different severities of diarrhea reasonably well. The effect of lumretuzumab and pertuzumab was well described by an Emax function indicating an increased rate of transition from moderate to mild or more severe diarrhea with higher doses. The concentration needed to trigger or worsen diarrhea episodes was estimated to be 120-fold lower in combination therapy compared to monotherapy, suggesting strong synergy between the two monoclonal antibodies. The prophylactic effect of loperamide in a subset of patients was also well captured by the model with a clear tendency to reduce the occurrence of diarrhea events.
Conclusions
This work shows that PK-toxicity modeling provides insight into how the severity of key adverse events evolves over time and highlights the potential use to support decision making in drug development.





Similar content being viewed by others
References
Campbell MR, Amin D, Moasser MM (2010) HER3 comes of age: new insights into its functions and role in signaling, tumor biology, and cancer therapy. Clin Cancer Res 16:1373–1383. https://doi.org/10.1158/1078-0432.CCR-09-1218
Schneeweiss A, Chia S, Hickish T, Harvey V, Eniu A, Hegg R, Tausch C, Seo JH, Tsai Y-F, Ratnayake J, McNally V, Ross G, Cortés J (2013) Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA). Ann Oncol 24:2278–2284. https://doi.org/10.1093/annonc/mdt182
Gianni L, Pienkowski T, Im Y-H, Tseng L-M, Liu M-C, Lluch A, Staroslawska E, de la Haba-Rodriguez J, Im S-A, Pedrini J, Poirier B, Morandi P, Semiglazov V, Srimunnimit V, Bianchi GV, Magazzu D, McNally V, Douthwaite H, Ross G, Valagussa P (2016) 5-year analysis of neoadjuvant pertuzumab and trastuzumab in patients with locally advanced, infl ammatory, or early-stage HER2-positive breast cancer (NeoSphere): a multicentre, open-label, phase 2 randomised trial. Lancet Onco 17:791–800. https://doi.org/10.1016/S1470-2045(16)00163-7
Von Minckwitz G, Procter M, de Azambuja E, Zardavas D, Benyunes M, Viale G, Suter T, Arahmani A, Rouchet N, Clark E, Knott A, Lang I, Levy C, Yardley DA, Bines J, Gelber RD, Piccart M, Baselga J (2017) Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer. N Engl J Med 377:122–131. https://doi.org/10.1056/NEJMoa1703643
Swain SM, Baselga J, Kim S-B, Ro J, Semiglazov V, Campone M, Ciruelos E, Ferrero J-M, Schneeweiss A, Heeson S, Clark E, Ross G, Benyunes M, Cortés J (2015) Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med 372:724–734. https://doi.org/10.1056/NEJMoa1413513
Collins D, Jacob W, Cejalvo JM, Ceppi M, James I, Hasmann M, Crown J, Cervantes A, Weisser M, Bossenmaier B (2017) Direct estrogen receptor (ER)/HER family crosstalk mediating sensitivity to lumretuzumab and pertuzumab in ER + breast cancer. PLoS ONE 12:e0177331. https://doi.org/10.1371/journal.pone.0177331
Way TD, Lin JK (2005) Role of HER2/HER3 co-receptor in breast carcinogenesis. Future Oncol 1:841–849. https://doi.org/10.2217/14796694.1.6.841
Meneses-Lorente G, Friess T, Kolm I, Hölzlwimmer G, Bader S, Meille C, Thomas M, Bossenmaier B (2015) Preclinical pharmacokinetics, pharmacodynamics, and efficacy of RG7116: a novel humanized, glycoengineered anti-HER3 antibody. Cancer Chemother Pharmacol 75:837–850. https://doi.org/10.1007/s00280-015-2697-8
Meulendijks D, Jacob W, Martinez-Garcia M, Taus A, Lolkema MP, Voest EE, Langenberg MHG, Kanonnikoff TF, Cervantes A, De Jonge MJ, Sleijfer S, Soerensen MM, Thomas M, Ceppi M, Meneses-Lorente G, James I, Adessi C, Michielin F, Abiraj K, Bossenmaier B, Schellens JHM, Weisser M, Lassen UN (2016) First-in-human phase I study of lumretuzumab, a glycoengineered humanized anti-HER3 monoclonal antibody, in patients with metastatic or advanced HER3-positive solid tumors. Clin Cancer Res 22:877–885. https://doi.org/10.1158/1078-0432.CCR-15-1683
Schneeweiss A et al. (2018) Phase Ib study evaluating safety and clinical activity of the anti-HER3 antibody lumretuzumab combined with the anti-HER2 antibody pertuzumab and paclitaxel in HER3-positive, HER2-low metastatic breast cancer. Invest New Drugs. https://doi.org/10.1007/s10637-018-0562-4. (Epub ahead of print)
Gianni L, Llado A, Bianchi G, Cortes J, Kellokumpu-Lehtinen P-L, Cameron D-A, Salvagni S, Wardley A, Goeminne DA, Hersberger V, Baselga J (2010) Open-label, phase II, multicenter, randomized study of the efficacy and safety of two dose levels of pertuzumab, a human epidermal growth factor receptor 2 dimerization inhibitor, in patients with human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol 28:1131–1137. https://doi.org/10.1200/JCO.2009.24.1661
Jones SE, Erban J, Overmoyer B, Budd GT, Hutchins K, Lower E, Laufman L, Sundaram S, Urba WJ, Pritchard KI, Mennel R, Richards D, Olsen S, Meyers ML, Ravdin PM (2005) Randomized phase III study of docetaxel compared with paclitaxel in metastatic breast cancer. J Clin Oncol 23:5542–5551. https://doi.org/10.1200/jco.2005.02.027
Koch G, Schropp J, Jusko WJ (2016) Assessment of non-linear combination effect terms for drug -drug interactions. J PK PD 43:461–479. https://doi.org/10.1007/s10928-016-9490-0
Jonker DM, Visser SA, van der Graaf PH, Voskuyl RA, Danhof M (2005) Towards a mechanism-based analysis of pharmacodynamic drug-drug interactions in vivo. Pharmacol Ther 106:1–18. https://doi.org/10.1016/j.pharmthera.2004.10.014
Meneses-Lorente G, McIntyre C, Hsu JC, Thomas M, Jacob W, Adessi C, Weisser M (2017) Accelerating drug development by efficiently using emerging PK/PD data from an adaptable entry-into-human trial: example of lumretuzumab. Cancer Chemother Pharmacol 79:1239–1247. https://doi.org/10.1007/s00280-017-3328-3
Garg A, Quartino A, Li J, Jin J, Wada DR, Li H, Cortes J, McNally V, Ross G, Visich J, Lum B (2014) Population pharmacokinetic and covariate analysis of pertuzumab, a HER2-targeted monoclonal antibody, and evaluation of a fixed, non-weight-based dose in patients with a variety of solid tumors. Cancer Chemother Pharmacol 74:819–829. https://doi.org/10.1007/s00280-014-2560-3
Bishop YM, Fienberg SE, Holland PW (1975) Discrete multivariate analysis. MIT Press, Cambridge
Schindler E, Karlsson MO (2017) A Minimal continuous-time Markov pharmacometric model. AAPS J 19:1424–1435. https://doi.org/10.1208/s12248-017-0109-1
Andersen PK, Borgan P, Gill RD, Keiding N (1993) Statistical models based on counting—processes—processes springer series in statistics. Springer, NewYork
Pilla Reddy V, Petersson KJ, Suleiman AA, Vermeulen A, Proost JH, Friberg LE (2012) Pharmacokinetic-pharmacodynamic modeling of severity levels of extrapyramidal side effects with Markov elements. CPT: Pharmacometrics Syst Pharmacol 1:1–9. https://doi.org/10.1038/psp.2012.9
Suleiman AA, Frechen S, Scheffler M, Zander T, Nogova L, Kocher M, Jaehde U, Wolf J, Fuhr U (2015) A modeling and simulation framework for adverse events in erlotinib-treated non-small-cell lung cancer Patients. AAPS J 17:1483–1491. https://doi.org/10.1208/s12248-015-9815-8
Minto CF, Schnider TW, Short TG, Gregg KM, Gentilini A, Shafer SL (2000) Response surface model for anesthetic drug interactions. Anesthesiology 92:1603–1616
Greco WR, Bravo G, Parsons JC (1995) The search for synergy: a critical review from a response surface perspective. Pharmacol Rev 47:331–385
Pawaskar DK, Straubinger RM, Fetterly GJ, Hylander BH, Repasky EA, Ma WW, Jusko WJ (2013) Synergistic interactions between sorafenib and everolimus in pancreatic cancer xenografts in mice. Cancer Chemother Pharmacol 71:1231–1240. https://doi.org/10.1007/s00280-013-2117-x
Keizer RJ, Karlsson MO, Hooker A (2013) Modeling and simulation workbench for NONMEM: tutorial on Pirana, PsN, and Xpose. CPT Pharmacometrics Syst Pharmacol 2: 1–9. https://doi.org/10.1038/psp.2013.24
Acknowledgements
The authors thank Dr Ahmed Suleiman (Abbvie Inc.) and Dr. Lena Friberg (Associate professor, Uppsala University) for their valuable inputs in the implementation of the compartmental continuous-time Markov model. We also thank Dr. Francesca Michielin of Roche for having provided the diarrhea dataset and made suggestions to the modeling efforts as well as Dr. Jonathan Wagg of Roche for his input in the overall work. We would like to thank the BP27771, BO16934, and BP28752 study teams for their assistance and support in the preparation of this manuscript. Finally, we would like to express our gratitude to the editor and two anonymous reviewers for their insightful and constructive comments, which made us greatly improve the presentation and content of the article.
Funding
The studies were funded by Hoffmann-La Roche Ltd.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors are employees of F. Hoffmann-La Roche Ltd at the time of data collection and analysis.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the studies.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Xu, C., Ravva, P., Dang, J.S. et al. A continuous-time multistate Markov model to describe the occurrence and severity of diarrhea events in metastatic breast cancer patients treated with lumretuzumab in combination with pertuzumab and paclitaxel. Cancer Chemother Pharmacol 82, 395–406 (2018). https://doi.org/10.1007/s00280-018-3621-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-018-3621-9