Abstract
Purpose
This study aimed at evaluating if pharmacokinetic and pharmacodynamic data from the first few patients treated with an investigational monoclonal antibody in a dose-escalation study can be used to guide the early initiation of potentially more efficacious combination regimens.
Methods
Emerging pharmacokinetic and pharmacodynamic data from the first nine patients treated with lumretuzumab (a glycoengineered anti-HER3 monoclonal antibody) monotherapy at doses from 100 to 400 mg q2w were used along with a pharmacokinetic model that incorporated target-mediated drug disposition to guide the selection of the starting dose for use in combination regimens.
Results
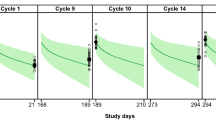
The dose-escalation study investigated lumretuzumab doses up to 2000 mg q2w and a maximum tolerated dose was not reached. However, the model described in this report predicted linear lumretuzumab pharmacokinetics and >95% target saturation at doses ≥400 mg q2w. These data, along with safety data, contributed to the decision to begin dose-escalation studies in combination with cetuximab and erlotinib using a starting dose of 400 mg lumretuzumab. Pharmacokinetic data from patients treated with lumretuzumab 400–2000 mg q2w in combination regimens were consistent with the model predictions.
Conclusion
PK/PD modelling of emerging clinical data might accelerate development programs by enabling additional parts of a trial to commence before completion of the monotherapy part. The dose and schedule of lumretuzumab were optimised for concomitant therapy at doses substantially below the highest dose investigated.






Similar content being viewed by others
References
Barrett JS, Gupta M, Mondick JT (2007) Model-based drug development applied to oncology. Expert Opin Drug Discov 2:185–209
Dostalek M, Gardner I, Gurbaxani BM, Rose RH, Chetty M (2013) Pharmacokinetics, pharmacodynamics and physiologically-based pharmacokinetic modelling of monoclonal antibodies. Clin Pharmacokinet 52:83–124
Mager DE (2006) Target-mediated drug disposition and dynamics. Biochem Pharmacol 72:1–10
Mager DE, Jusko WJ (2001) General pharmacokinetic model for drugs exhibiting target-mediated drug disposition. J Pharmacokinet Pharmacodyn 28:507–532
Cao Y, Jusko WJ (2014) Incorporating target-mediated drug disposition in a minimal physiologically-based pharmacokinetic model for monoclonal antibodies. J Pharmacokinet Pharmacodyn 41:375–387
Luu KT, Bergqvist S, Chen E, Hu-Lowe D, Kraynov E (2012) A model-based approach to predicting the human pharmacokinetics of a monoclonal antibody exhibiting target-mediated drug disposition. J Pharmacol Exp Ther 341:702–708
Amin DN, Campbell MR, Moasser MM (2010) The role of HER3, the unpretentious member of the HER family, in cancer biology and cancer therapeutics. Semin Cell Dev Biol 21:944–950
Mirschberger C, Schiller CB, Schräml M, Dimoudis N, Friess T, Gerdes CA, Reiff U, Lifke V, Hoelzlwimmer G, Kolm I, Hopfner K-P, Niederfellner G, Bossenmaier B (2013) RG7116, a therapeutic antibody that binds the inactive HER3 receptor and is optimized for immune effector activation. Can Res 73:5183–5194
Meneses-Lorente G, Friess T, Kolm I, Holzlwimmer G, Bader S, Meille C, Thomas M, Bossenmaier B (2015) Preclinical pharmacokinetics, pharmacodynamics, and efficacy of RG7116: a novel humanized, glycoengineered anti-HER3 antibody. Cancer Chemother Pharmacol 75:837–850
Meulendijks D, Jacob W, Martinez-Garcia M, Taus A, Lolkema MP, Voest EE, Langenberg MH, Fleitas Kanonnikoff T, Cervantes A, De Jonge MJ, Sleijfer S, Soerensen MM, Thomas M, Ceppi M, Meneses-Lorente G, James I, Adessi C, Michielin F, Abiraj K, Bossenmaier B, Schellens JH, Weisser M, Lassen UN (2016) First-in-human phase I study of lumretuzumab, a glycoengineered humanized anti-HER3 monoclonal antibody, in patients with metastatic or advanced HER3-positive solid tumors. Clin Can Res 22:877–885
Lassen UN, Cervantes-Ruiperez A, Fleitas T, Meulendijks D, Schellens JH, Lolkema MP, De Jonge MJ, Sleijfer S, Mau Soerensen M, Taus A, Adessi C, Keelarar A, Michielin F, Bossenmaier B, Meneses-Lorente G, James I, Jacob W, Weisser M, Marti M (2014) Phase Ib trial trial of RG7116, a glycoengineered monoclonal antibody targeting HER3, in combination with cetuximab or erlotinib in patients with advanced/metastatic tumors of epithelial cell origin expressing HER3 protein. Ann Oncol 25(Suppl_4):abstract 4440
Meulendijks D, Lolkema MPJK, Voest EE, De Jonge MJ, Sleijfer S, Schellens JHM, Fleitas T, Cervantes-Ruiperez A, Martinez-Garcia M, Taus A, Mau Sorensen M, Thomas M, Meneses-Lorente G, Adessi C, Di Scala L, Keelarar A, Jacob W, Lassen UN (2013) A first-in-human trial of RG7116, a glycoengineered monoclonal antibody targeting HER3, in patients with advanced/metastatic tumors of epithelial cell origin expressing HER3 protein. J Clin Oncol 31(15_suppl):abstract 2522
Acknowledgements
The authors would like to thank the entire BP27771 study team at Penzberg for their assistance in the preparation of this manuscript. Support for third-party writing assistance for this article, furnished by Jamie Ashman, PhD, was provided by Prism Ideas and funded by Roche Diagnostics GmbH, Penzberg.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by Roche Diagnostics GmbH, Penzberg, Germany.
Conflict of interest
All authors are employees of F. Hoffmann-La Roche Ltd.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Meneses-Lorente, G., McIntyre, C., Hsu, J.C. et al. Accelerating drug development by efficiently using emerging PK/PD data from an adaptable entry-into-human trial: example of lumretuzumab. Cancer Chemother Pharmacol 79, 1239–1247 (2017). https://doi.org/10.1007/s00280-017-3328-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-017-3328-3