Abstract
Purpose
Lenalidomide disease-specific toxicity profiles and potentially life-threatening adverse events support the consideration of diversity in starting doses. The aim of this study was to conduct a population pharmacokinetic analysis of lenalidomide in multiple myeloma patients to identify and evaluate non-studied covariates that could be used for dose individualization.
Methods
Blood samples were collected from 15 multiple myeloma patients. Nonlinear mixed-effects modeling was used to develop a population pharmacokinetic model and perform covariate analysis. The developed model was used to simulate dose schedules in order to explore the need of different dosing regimens in patients with different covariate values.
Results
The data were accurately described by a one-compartment model with first-order elimination. Absorption was best described using three transit compartments. Creatinine clearance and body surface area were identified as covariates affecting apparent clearance and apparent volume of distribution, respectively. Simulations revealed that lower starting doses than the standard 25 mg/daily could be used in patients with body surface area below 1.8 m2 and even higher doses might be necessary for patients with normal renal function and large body surface area.
Conclusions
This study identified creatinine clearance and body surface area as covariates that have a clinically relevant impact on lenalidomide pharmacokinetics using population pharmacokinetics. In addition, the developed population pharmacokinetic model can be used to individualize lenalidomide dose in multiple myeloma patients, taking into account not only creatinine clearance but also body surface area.





Similar content being viewed by others
References
Kotla V, Goel S, Nischal S et al (2009) Mechanism of action of lenalidomide in hematological malignancies. J Hematol Oncol 2:36. doi:10.1186/1756-8722-2-36
Quach H, Ritchie D, Stewart AK et al (2010) Mechanism of action of immunomodulatory drugs (IMiDS) in multiple myeloma. Leukemia 24:22–32. doi:10.1038/leu.2009.236
Richardson PG, Schlossman RL, Weller E et al (2002) Immunomodulatory drug CC-5013 overcomes drug resistance and is well tolerated in patients with relapsed multiple myeloma. Blood 100:3063–3067. doi:10.1182/blood-2002-03-0996
Weber DM, Chen C, Niesvizky R et al (2007) Lenalidomide plus dexamethasone for relapsed multiple myeloma in North America. N Engl J Med 357:2133–2142. doi:10.1056/NEJMoa070596
Dimopoulos M, Spencer A, Attal M et al (2007) Lenalidomide plus dexamethasone for relapsed or refractory multiple myeloma. N Engl J Med 357:2123–2132. doi:10.1056/NEJMoa070594
Seiffert M (2014) Lenalidomide, an antiproliferative CLL drug. Blood 124:1545–1546. doi:10.1182/blood-2014-07-587360
Fowler NH, Davis RE, Rawal S et al (2014) Safety and activity of lenalidomide and rituximab in untreated indolent lymphoma: an open-label, phase 2 trial. Lancet Oncol 15:1311–1318. doi:10.1016/S1470-2045(14)70455-3
Bejar R, Steensma DP (2014) Recent developments in myelodysplastic syndromes. Blood 124:2793–2803. doi:10.1182/blood-2014-04-522136
Rao KV (2007) Lenalidomide in the treatment of multiple myeloma. Am J Health Syst Pharm 64:1799–1807. doi:10.2146/ajhp070029
Andritsos LA, Johnson AJ, Lozanski G et al (2008) Higher doses of lenalidomide are associated with unacceptable toxicity including life-threatening tumor flare in patients with chronic lymphocytic leukemia. J Clin Oncol 26:2519–2525. doi:10.1200/JCO.2007.13.9709
Palumbo A, Falco P, Corradini P et al (2007) Melphalan, prednisone, and lenalidomide treatment for newly diagnosed myeloma: a report from the GIMEMA–Italian Multiple Myeloma Network. J Clin Oncol 25:4459–4465. doi:10.1200/JCO.2007.12.3463
Berg SL, Cairo MS, Russell H et al (2011) Safety, pharmacokinetics, and immunomodulatory effects of lenalidomide in children and adolescents with relapsed/refractory solid tumors or myelodysplastic syndrome: a Children’s Oncology Group Phase I Consortium report. J Clin Oncol 29:316–323. doi:10.1200/JCO.2010.30.8387
Grzasko N, Morawska M, Hus M (2015) Optimizing the treatment of patients with multiple myeloma and renal impairment. Clin Lymphoma Myeloma Leuk 15:187–198. doi:10.1016/j.clml.2014.09.012
Kado Y, Kitazawa F, Tsujimoto M et al (2015) Prediction of the lenalidomide toxicity and its therapeutic efficacy in Japanese multiple myeloma patients by measuring its plasma concentration. Blood 126:3700
EMA (2007) Revlimid, INN-lenalidomide EPAR scientific discussion. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Scientific_Discussion/human/000717/WC500056022.pdf. Accessed 25 Dec 2016
Chen N, Lau H, Kong L et al (2007) Pharmacokinetics of lenalidomide in subjects with various degrees of renal impairment and in subjects on hemodialysis. J Clin Pharmacol 47:1466–1475. doi:10.1177/0091270007309563
Klein U, Neben K, Hielscher T et al (2011) Lenalidomide in combination with dexamethasone: effective regimen in patients with relapsed or refractory multiple myeloma complicated by renal impairment. Ann Hematol 90:429–439. doi:10.1007/s00277-010-1080-4
Hou J, Du X, Jin J et al (2013) A multicenter, open-label, phase 2 study of lenalidomide plus low-dose dexamethasone in Chinese patients with relapsed/refractory multiple myeloma: the MM-021 trial. J Hematol Oncol 6:41. doi:10.1186/1756-8722-6-41
Iida S, Chou T, Okamoto S et al (2010) Lenalidomide plus dexamethasone treatment in Japanese patients with relapsed/refractory multiple myeloma. Int J Hematol 92:118–126. doi:10.1007/s12185-010-0624-7
Ette E, Zhou S, Weiss D, Palmisano M (2013) Population pharmacokinetics and exposure-safety of lenalidomide in patients with multiple myeloma, myelodysplastic syndromes and mantle cell lymphoma. Blood 122:3234
Shida S, Takahashi N, Miura M et al (2014) A limited sampling model to estimate exposure to lenalidomide in multiple myeloma patients. Ther Drug Monit 36:505–509. doi:10.1097/FTD.0000000000000034
Guglieri-Lopez B, Perez-Pitarch A, Martinez-Gomez MA et al (2016) A wide linearity range method for the determination of lenalidomide in plasma by high-performance liquid chromatography: application to pharmacokinetic studies. J Lab Autom. doi:10.1177/2211068216636570
Beal S, Sheiner L, Boeckmann A (2006) NONMEM users guide (1989–2006). Icon Development Solutions, Ellicott City, USA
Lindbom L, Pihlgren P, Jonsson N (2005) PsN-toolkit—a collection of computer intensive statistical methods for non-linear mixed effect modeling using NONMEM. Comput Methods Programs Biomed 79:241–257. doi:10.1016/j.cmpb.2005.04.005
Keizer RJ, van Benten M, Beijnen JH et al (2011) Pirana and PCluster: a modeling environment and cluster infrastructure for NONMEM. Comput Methods Programs Biomed 101:72–79. doi:10.1016/j.cmpb.2010.04.018
Keizer RJ, Jansen RS, Rosing H et al (2015) Incorporation of concentration data below the limit of quantification in population pharmacokinetic analyses. Pharmacol Res Perspect 3:e00131. doi:10.1002/prp2.131
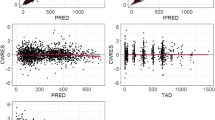
Bergstrand M, Hooker AC, Wallin JE, Karlsson MO (2011) Prediction-corrected visual predictive checks for diagnosing nonlinear mixed-effects models. AAPS J 13:143–151. doi:10.1208/s12248-011-9255-z
Chen N, Zhou S, Palmisano M (2016) Clinical pharmacokinetics and pharmacodynamics of lenalidomide. Clin Pharmacokinet. doi:10.1007/s40262-016-0432-1
Tunblad K, Lindbom L, McFadyen L et al (2008) The use of clinical irrelevance criteria in covariate model building with application to dofetilide pharmacokinetic data. J Pharmacokinet Pharmacodyn 35:503–526. doi:10.1007/s10928-008-9099-z
Chen N, Kasserra C, Reyes J et al (2012) Single-dose pharmacokinetics of lenalidomide in healthy volunteers: dose proportionality, food effect, and racial sensitivity. Cancer Chemother Pharmacol 70:717–725. doi:10.1007/s00280-012-1966-z
Ribbing J, Jonsson EN (2004) Power, selection bias and predictive performance of the Population Pharmacokinetic Covariate Model. J Pharmacokinet Pharmacodyn 31:109–134
Myeloma incidence statistics—Cancer Research UK. http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/myeloma/incidence. Accessed 17 Aug 2016
Acknowledgements
We thank patients with multiple myeloma for participating in this study and healthcare staff from Pharmacy and Hematology Department of Doctor Peset University Hospital for their support.
Funding
This work was supported by the Foundation for the Promotion of Health and Biomedical Research of the Valencian Community (FISABIO).
Author contribution
BG-L performed the data analysis and wrote the manuscript. AP-P, DJM, BP-O, MC-M, H-JG and MM-S contributed in the data analysis and revised the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Beatriz Guglieri-López, Alejandro Pérez-Pitarch, Dirk Jan Moes, Begoña Porta-Oltra, Mónica Climente-Martí, Henk-Jan Guchelaar and Matilde Merino-Sanjuán have no conflicts of interest that might be relevant to the content of this manuscript.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Electronic supplementary material
Below is the link to the electronic supplementary material.
280_2016_3228_MOESM3_ESM.tiff
Schematic representation of the pharmacokinetic model for lenalidomide. A linear one-compartment model with first-order absorption and elimination, including three transit compartments to describe the absorption phase (TIFF 1521 kb)
Rights and permissions
About this article
Cite this article
Guglieri-López, B., Pérez-Pitarch, A., Moes, D.J.A.R. et al. Population pharmacokinetics of lenalidomide in multiple myeloma patients. Cancer Chemother Pharmacol 79, 189–200 (2017). https://doi.org/10.1007/s00280-016-3228-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-016-3228-y