Abstract
Background
The California Cancer Consortium completed a phase I trial of E7389 (eribulin mesylate), an analog of the marine natural product halichondrin B. This trial was to determine the pharmacodynamics, pharmacokinetics, and MTD of E7389 administered by bolus injection weekly for 3 weeks out of four.
Methods
This trial included a rapid titration design. Real-time pharmacokinetics were utilized to guide dose escalation. Initially, single-patient cohorts were enrolled with intra- and inter-patient dose doubling. The second phase was a standard 3 + 3 dose escalation schedule. At the MTD, a cohort of patients was enrolled for target validation studies (separate manuscript). The starting dose was 0.125 mg/m2, and doses were doubled within and between patients in the first phase. Blood and urine sampling for E7389 pharmacokinetics was performed on doses 1 and 3 of cycle 1. Levels were determined using a LC/MS/MS assay.
Results
Forty patients were entered. Thirty-eight were evaluable for toxicity and 35 for response. The rapid escalation ended with a grade 3 elevation of alkaline phosphatase at 0.5 mg/m2/week. The second phase ended at 2.0 mg/m2/week with dose-limiting toxicities of grades 3 and 4 febrile neutropenia. Other toxicities included hypoglycemia, hypophosphatemia, and fatigue. The MTD was 1.4 mg/m2/week. Responses included four partial responses (lung cancer [2], urothelial [1], and melanoma [1]).
Conclusions
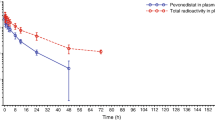
E7389 was well tolerated in this trial with the major toxicity being myelosuppression. PD shows that E7389 induces significant morphologic changes (bundle formation) in the microtubules of peripheral blood mononuclear cells and tumor cells in vivo. The data suggest that lower intra-tumoral levels of β-tubulin III or higher intra-tumoral levels of MAP4 may correlate with response to E7389, while lower intra-tumoral levels of stathmin may be associated with progression. PK data reveal that E7389 exhibits a tri-exponential elimination from the plasma of patients receiving a rapid i.v. infusion. At sub-toxic doses, plasma concentrations of E7389 are maintained well above the levels required for activity in vitro for >72 h.




Similar content being viewed by others
References
Margolin K, Synold T, Longmate J, Doroshow JH (2001) Methodologic guidelines for the design of high-dose chemotherapy regimens. Biol Blood Marrow Transpl 7(8):414–432
Kraljevic S, Sedic M, Scott M, Gehrig P, Schlapbach R, Pavelic K (2006) Casting light on molecular events underlying anti-cancer drug treatment: what can be seen from the proteomics point of view? Cancer Treat Rev 32(8):619–629
Collins I, Workman P (2006) New approaches to molecular cancer therapeutics. Nat Chem Biol 2(12):689–700
Towle MJ, Salvato KA, Budrow J, Wels BF, Kuznetsov G, Aalfs KK, Welsh S, Zheng W, Seletsk BM, Palme MH, Habgood GJ, Singer LA, DiPietro LV, Wang Y, Chen JJ, Quincy DA, Davis A, Yoshimatsu K, Kishi Y, Yu MJ, Littlefield BA (2001) In vitro and in vivo anticancer activities of synthetic macrocyclic ketone analogues of halichondrin B. Cancer Res 61(3):1013–1021
Jordan MA, Kamath K, Manna T, Okouneva T, Miller HP, Davis C, Littlefield BA, Wilson L (2005) The primary antimitotic mechanism of action of the synthetic halichondrin E7389 is suppression of microtubule growth. Mol Cancer Ther 4(7):1086–1095
Perez EA (2009) Microtubule inhibitors: differentiating tubulin-inhibiting agents based on mechanisms of action, clinical activity, and resistance. Mol Cancer Ther 8(8):2086–2095
Jimeno A (2009) Eribulin: rediscovering tubulin as an anticancer target. Clin Cancer Res 15(12):3903–3905
Okouneva T, Azarenko O, Wilson L, Littlefield BA, Jordan MA (2008) Inhibition of centromere dynamics by eribulin (E7389) during mitotic metaphase. Mol Cancer Ther 7(7):2003–2011
Hirata Y, Uemura D (1986) Halichondrins-antitumor polyether macrolides from murine sponges. Pure Appl Chem 58:701–710
Bai R, Cichacz ZA, Herald CL, Pettit GR, Hamel E (1993) Spongistatin 1, a highly cytotoxic, sponge-derived, marine natural product that inhibits mitosis, microtubule assembly, and the binding of vinblastine to tubulin. Mol Pharmacol 44(4):757–766
Bai RL, Paull KD, Herald CL, Malspeis L, Pettit GR, Hamel E (1991) Halichondrin B and homohalichondrin B, marine natural products binding in the vinca domain of tubulin. Discovery of tubulin-based mechanism of action by analysis of differential cytotoxicity data. J Biol Chem 266(24):15882–15889
Fodstad O, Breistol K, Pettit GR, Shoemaker RH, Boyd MR (1996) Comparative antitumor activities of halichondrins and vinblastine against human tumor xenografts. J Exp Ther Oncol 1(2):119–125
Luduena RF, Roach MC, Prasad V, Pettit GR (1993) Interaction of halichondrin B and homohalichondrin B with bovine brain tubulin. Biochem Pharmacol 45(2):421–427
Hamel E (1992) Natural products which interact with tubulin in the vinca domain: maytansine, rhizoxin, phomopsin A, dolastatins 10 and 15 and halichondrin B. Pharmacol Ther 55(1):31–51
Smith JA, Wilson L, Azarenko O, Zhu X, Lewis BM, Littlefield BA, Jordan MA (2010) Eribulin binds at microtubule ends to a single site on tubulin to suppress dynamic instability. Biochemistry 49(6):1331–1337
Kuznetsov G, Towle MJ, Cheng H, Kawamura T, TenDyke K, Liu D, Kishi Y, Yu MJ, Littlefield BA (2004) Induction of morphological and biochemical apoptosis following prolonged mitotic blockage by halichondrin B macrocyclic ketone analog E7389. Cancer Res 64(16):5760–5766
Simon R, Freidlin B, Rubinstein L, Arbuck SG, Collins J, Christian MC (1997) Accelerated titration designs for phase I clinical trials in oncology. J Natl Cancer Inst 89(15):1138–1147
Towle MJ, Agoulnik S, Kuznetsov G, TenDyke K, Reardon C, Cheng H, Zheng W, Seletsky BM, Palme MH, Kishi Y, Lewis MD, Yu MJ, Littlefield BA (2003) In vivo efficacy of E7389, a synthetic analog of the marine sponge antitubulin agent halichondrin B, against human tumor xenografts under monotherapy and combination therapy conditions. Proc Am Assoc Cancer Res 44:628
McDaid HM, Mani S, Shen HJ, Muggia F, Sonnichsen D, Horwitz SB (2002) Validation of the pharmacodynamics of BMS-247550, an analogue of epothilone B, during a phase I clinical study. Clin Cancer Res 8(7):2035–2043
Simmons TL, Andrianasolo E, McPhail K, Flatt P, Gerwick WH (2005) Marine natural products as anticancer drugs. Mol Cancer Ther 4(2):333–342
Alley M, Dykes D, Waud W, Pacula-Cox D, Munro M, Newman D, Sausville E (1998) Efficacy evaluations of halichondrin B in selected xenografts. Proc Am Assoc Cancer Res 26:A1545
Goel S, Mita AC, Mita M, Rowinsky EK, Chu QS, Wong N, Desjardins C, Fang F, Jansen M, Shuster DE, Mani S, Takimoto CH (2009) A phase I study of eribulin mesylate (E7389), a mechanistically novel inhibitor of microtubule dynamics, in patients with advanced solid malignancies. Clin Cancer Res 15(12):4207–4212
Tan AR, Rubin EH, Walton DC, Shuster DE, Wong YN, Fang F, Ashworth S, Rosen LS (2009) Phase I study of eribulin mesylate administered once every 21 days in patients with advanced solid tumors. Clin Cancer Res 15(12):4213–4219
Giannakakou P, Poy G, Zhan Z, Knutsen T, Blagosklonny MV, Fojo T (2000) Paclitaxel selects for mutant or pseudo-null p53 in drug resistance associated with tubulin mutations in human cancer. Oncogene 19(27):3078–3085
Day BW (2000) Mutants yield a pharmacophore model for the tubulin–paclitaxel binding site. Trends Pharmacol Sci 21(9):321–324
Giannakakou P, Gussio R, Nogales E, Downing KH, Zaharevitz D, Bollbuck B, Poy G, Sackett D, Nicolaou KC, Fojo T (2000) A common pharmacophore for epothilone and taxanes: molecular basis for drug resistance conferred by tubulin mutations in human cancer cells. Proc Natl Acad Sci USA 97(6):2904–2909
Monzo M, Rosell R, Sanchez JJ, Lee JS, O’Brate A, Gonzalez-Larriba JL, Alberola V, Lorenzo JC, Nunez L, Ro JY, Martin C (1999) Paclitaxel resistance in non-small-cell lung cancer associated with beta-tubulin gene mutations. J Clin Oncol 17(6):1786–1793
Kavallaris M, Tait AS, Walsh BJ, He L, Horwitz SB, Norris MD, Haber M (2001) Multiple microtubule alterations are associated with Vinca alkaloid resistance in human leukemia cells. Cancer Res 61(15):5803–5809
Gan PP, Pasquier E, Kavallaris M (2007) Class III {beta}-tubulin mediates sensitivity to chemotherapeutic drugs in non small cell lung cancer. Cancer Res 67(19):9356–9363
Tommasi S, Mangia A, Lacalamita R, Bellizzi A, Fedele V, Chiriatti A, Thomssen C, Kendzierski N, Latorre A, Lorusso V, Schittulli F, Zito F, Kavallaris M, Paradiso A (2007) Cytoskeleton and paclitaxel sensitivity in breast cancer: the role of beta-tubulins. Int J Cancer 120(10):2078–2085
Mozzetti S, Ferlini C, Concolino P, Filippetti F, Raspaglio G, Prislei S, Gallo D, Martinelli E, Ranelletti FO, Ferrandina G, Scambia G (2005) Class III beta-tubulin overexpression is a prominent mechanism of paclitaxel resistance in ovarian cancer patients. Clin Cancer Res 11(1):298–305
Martello LA, Verdier-Pinard P, Shen HJ, He L, Torres K, Orr GA, Horwitz SB (2003) Elevated levels of microtubule destabilizing factors in a Taxol-resistant/dependent A549 cell line with an alpha-tubulin mutation. Cancer Res 63(6):1207–1213
Alli E, Bash-Babula J, Yang JM, Hait WN (2002) Effect of stathmin on the sensitivity to antimicrotubule drugs in human breast cancer. Cancer Res 62(23):6864–6869
Burkhart CA, Kavallaris M, Band HS (2001) The role of beta-tubulin isotypes in resistance to antimitotic drugs. Biochim Biophys Acta 1471(2):O1–O9
Cortes J, O’Shaughnessy J, Loesch D, Blum JL, Vahdat LT, Petrakova K, Chollet P, Manikas A, Dieras V, Delozier T, Vladimirov V, Cardoso F, Koh H, Bougnoux P, Dutcus CE, Seegobin S, Mir D, Meneses N, Wanders J, Twelves C (2011) Eribulin monotherapy versus treatment of physician’s choice in patients with metastatic breast cancer (EMBRACE): a phase 3 open-label randomised study. Lancet 377(9769):914–923
Scarpace SL (2012) Eribulin mesylate (E7389): review of efficacy and tolerability in breast, pancreatic, head and neck, and non-small cell lung cancer. Clin Ther 34(7):1467–1473
Gitlitz BJ, Tsao-Wei DD, Groshen S, Davies A, Koczywas M, Belani CP, Argiris A, Ramalingam S, Vokes EE, Edelman M, Hoffman P, Ballas MS, Liu SV, Gandara DR (2012) A phase II study of halichondrin B analog eribulin mesylate (E7389) in patients with advanced non-small cell lung cancer previously treated with a taxane: a California Cancer Consortium trial. J Thorac Oncol 7(3):574–578
Acknowledgments
This study was supported in part by National Cancer Institute Grants P30CA033572, UM1CA186717, U01CA062505, and M01RR000043.
Author information
Authors and Affiliations
Corresponding author
Additional information
Reported in part in Proc. Amer. Soc. Clin. Oncol. 22:A575, 2003, and 23:A3036, 2005, and in Proc. Amer. Assoc. Cancer Res. 44:A5344, 2003.
Rights and permissions
About this article
Cite this article
Morgan, R.J., Synold, T.W., Longmate, J.A. et al. Pharmacodynamics (PD) and pharmacokinetics (PK) of E7389 (eribulin, halichondrin B analog) during a phase I trial in patients with advanced solid tumors: a California Cancer Consortium trial. Cancer Chemother Pharmacol 76, 897–907 (2015). https://doi.org/10.1007/s00280-015-2868-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-015-2868-7