Abstract
Purpose
To assess the feasibility of administering troxacitabine, an l-nucleoside analog that is not a substrate for deoxycytidine deaminase, in combination with cisplatin, to identify pharmacokinetic interactions, and to seek preliminary evidence of antitumor activity.
Methods
Patients with advanced solid malignancies were treated with cisplatin intravenously over an hour followed by troxacitabine intravenously over 30 min on day 1 every 28 days at the following cisplatin/troxacitabine (mg/m2) dose levels 50/4.8, 75/4.8, 50/6.4, 75/6.4, and 75/8.0. Plasma and urine sampling were performed to characterize the pharmacokinetic parameters of troxacitabine in combination with cisplatin.
Results
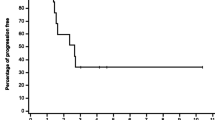
Thirty-one patients received 77 courses of cisplatin/troxacitabine at five dose levels. Grade 4 neutropenia lasting more than 5 days and/or grade 4 thrombocytopenia were consistently experienced by minimally pretreated (MP) and heavily pretreated (HP) patients at doses exceeding 75/6.4 and 50/4.8 mg/m2, respectively. Mean values for the volume of distribution at steady state and clearance of troxacitabine were 196–396 L and 7.2–9.8 L/h, respectively. A patient with metastatic non-small cell lung cancer experienced a 42% reduction in extent of disease for 6 months.
Conclusions
The combination of cisplatin and troxacitabine produces dose-limiting myelosuppression at lower doses of troxacitabine than single agent doses. The recommended phase II doses of cisplatin/troxacitabine are 75/6.4 and 50/4.8 mg/m2, every 4 weeks, for MP and HP patients, respectively. The addition of cisplatin did not substantially alter the pharmacokinetic behavior of troxacitabine.




Similar content being viewed by others
References
Grove KL, Guo X, Liu SH et al (1995) Anticancer activity of beta-l-dioxolane-cytidine, a novel nucleoside analogue with the unnatural l configuration. Cancer Res 55:3008–3011
Gourdeau H, Clarke ML, Ouellet F et al (2001) Mechanisms of uptake and resistance to troxacitabine, a novel deoxycytidine nucleoside analogue, in human leukemic and solid tumor cell lines. Cancer Res 61:7217–7224
Krishnan P, Fu Q, Lam W et al (2002) Phosphorylation of pyrimidine deoxynucleoside analog diphosphates: selective phosphorylation of l-nucleoside analog diphosphates by 3-phosphoglycerate kinase. J Biol Chem 277:5453–5459
Chou KM, Kukhanova M, Cheng YC (2000) A novel action of human apurinic/apyrimidinic endonuclease: excision of l-configuration deoxyribonucleoside analogs from the 3’ termini of DNA. J Biol Chem 275:31009–31015
Kukhanova M, Liu SH, Mozzherin D et al (1995) l- and d-enantiomers of 2′, 3′-dideoxycytidine 5′-triphosphate analogs as substrates for human DNA polymerases. Implications of the mechanism of toxicity. J Biol Chem 270:23055–23059
Giles FJ, Cortes JE, Baker SD et al (2001) Troxacitabine, a novel dioxolane nucleoside analog, has activity in patients with advanced leukemia. J Clin Oncol 19:762–771
de Bono JS, Stephenson J Jr, Baker SD et al (2002) Troxacitabine, an l-stereoisomeric nucleoside analog, on a five-times-daily schedule: a phase I and pharmacokinetic study in patients with advanced solid malignancies. J Clin Oncol 20:96–109
Belanger K, Moore M, Baker SD et al (2002) Phase I and pharmacokinetic study of novel l-nucleoside analog troxacitabine given as a 30-minute infusion every 21 days. J Clin Oncol 20:2567–2574
Lee CK, Rowinsky EK, Li J et al (2006) Population pharmacokinetics of troxacitabine, a novel dioxolane nucleoside analogue. Clin Cancer Res 12:2158–2165
Moufarij MA, Phillips DR, Cullinane C (2003) Gemcitabine potentiates cisplatin cytotoxicity and inhibits repair of cisplatin-DNA damage in ovarian cancer cell lines. Mol Pharmacol 63:862–869
van Moorsel CJ, Pinedo HM, Veerman G et al (1999) Mechanisms of synergism between cisplatin and gemcitabine in ovarian and non-small-cell lung cancer cell lines. Br J Cancer 80:981–990
Crul M, van Waardenburg RC, Bocxe S et al (2003) DNA repair mechanisms involved in gemcitabine cytotoxicity and in the interaction between gemcitabine and cisplatin. Biochem Pharmacol 65:275–282
Yang LY, Li L, Jiang H, Shen Y, Plunkett W (2000) Expression of ERCC1 antisense RNA abrogates gemicitabine-mediated cytotoxic synergism with cisplatin in human colon tumor cells defective in mismatch repair but proficient in nucleotide excision repair. Clin Cancer Res 6:773–781
Perrier D, Mayersohn M (1982) Noncompartmental determination of the steady-state volume of distribution for any mode of administration. J Pharm Sci 71:372–373
Cockcroft DW, Gault MH (1976) Prediction of creatinine clearance from serum creatinine
Baker SD, Verweij J, Rowinsky EK et al (2002) Role of body surface area in dosing of investigational anticancer agents in adults, 1991–2001. J Natl Cancer Inst 94:1883–1888
Shepherd FA, Burkes R, Cormier Y et al (1996) Phase I dose-escalation trial of gemcitabine and cisplatin for advanced non-small-cell lung cancer: usefulness of mathematic modeling to determine maximum-tolerable dose. J Clin Oncol 14:1656–1662
Giles FJ, Kantarjian HM, Cortes JE et al (2003) Adaptive randomized study of idarubicin and cytarabine versus troxacitabine and cytarabine versus troxacitabine and idarubicin in untreated patients 50 years or older with adverse karyotype acute myeloid leukemia. J Clin Oncol 21:1722–1727
Dent SF, Arnold A, Stewart DJ et al (2005) Phase II study of troxacitabine (BCH-4556) in patients with advanced non-small-cell lung cancer. Lung 183:265–272
Lapointe R, Letourneau R, Steward W et al (2005) Phase II study of troxacitabine in chemotherapy-naive patients with advanced cancer of the pancreas: gastrointestinal tumors. Ann Oncol 16:289–293
Jimeno A, Messersmith WA, Lee CK et al (2008) Phase I study of troxacitabine administered by continuous infusion in subjects with advanced solid malignancies. Ann Oncol 19:374–379
Roboz GJ, Giles FJ, Ritchie EK et al (2007) Phase I/II study of continuous-infusion troxacitabine in refractory acute myeloid leukemia. J Clin Oncol 25:10–15
Author information
Authors and Affiliations
Corresponding author
Additional information
C.-C. Lin and M. Beeram contributed equally to this work.
Rights and permissions
About this article
Cite this article
Lin, CC., Beeram, M., Rowinsky, E.K. et al. Phase I and pharmacokinetic study of cisplatin and troxacitabine administered intravenously every 28 days in patients with advanced solid malignancies. Cancer Chemother Pharmacol 65, 167–175 (2009). https://doi.org/10.1007/s00280-009-1020-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-009-1020-y