Abstract
Objective
4-Methyl-2,7-diamino-5,10-diphenyl-4,9-diazapyrenium hydrogensulfate (ADAP) is a potential antitumor compound because of its DNA and RNA intercalating ability. In this study, cellular uptake, intracellular distribution as well as mechanism of action, antitumor activity in vitro and toxicity in vivo of ADAP were investigated.
Methods
Based on the fluorescence properties of ADAP, its entry and distribution into live cells were analyzed by fluorescence microscopy. The in vitro antiproliferative activity was determined using MTT test. For screening of topoisomerase II-targeted effects of ADAP, the cell-free assay and immunoband depletion assay were used. Expression of the genes c-mos, c-N-ras, c-Ki-ras, c-H-ras, p53 and caspase 3 in Caco-2 cells treated with ADAP was examined by RT-PCR. Toxicity in vivo was determined using C3HHf/Bu Zgr/Hr mice treated by single or multiple doses of ADAP at a concentration of 25 mg/kg.
Results
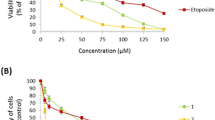
ADAP in μM concentrations entered into MIAPaCa-2 cell’s cytoplasm in 5 min and into nuclei in 60 min after administration. Intracellular distribution of ADAP depended on the period of treatment time. ADAP (0.1–100 μM) strongly inhibited the growth of both mouse (FsaR, SCCVII) and human tumor cells (HeLa, Caco-2, HT-29, MIAPaCa-2, HBL, HEp-2, SW620, MCF-7) compared to its weak cytotoxicity on controls and normal cells (WI38). Results of both topoisomerase II assays showed that ADAP is not a topoisomerase II poison. Expression of investigated genes was dependent on the incubation time, except for p53 and c-H-ras. Morphological changes in tissues and organs of mice were not observed. Results of patohistological analysis have been confirmed by hematological and clinical-chemical analysis of blood of treated and non-treated animals.
Conclusion
ADAP is a strongly bioactive compound with antitumor potential in vitro. The antitumor potential in vivo remains to be identified.








Similar content being viewed by others
References
Ferlay J, Parkin MD, Pisani P (1998) Globocan: Cancer incidence and mortality worldwide. International Agency for Research on Cancer, IARC Cancer Base, Lyon, France, pp 3
Howard CV, Newby JA (2004) Could the increase in cancer incidence be related to recent environmental changes. In: Nicolopoulou-Stamati P, Hens L, Howard CV, Van Larebeke L (eds) Cancer as an environmental disease. Kluwer, Dordrecht
Li Q, Xu W (2005) Novel anticancer targets in drug discovery in postgenomic age. Curr Med Chem Anticancer Agents 5:53–63
Lerman LS (1961) Structural considerations in the interaction of DNA and acridines. J Mol Biol 3:18–30
Demeunyck M, Bailly C, Wilson WD (2002) DNA and RNA binders: from small molecules to drugs. Wiley, New York
Silverman RB (2004) The organic chemistry of drug design and drug action, 2nd edn. Elsevier, San Diego
Taylor IW, Milthorpe BK (1980) An evaluation of DNA fluorochromes, staining techiques and analysis for flow cytometry. J Histochem Cytochem 28(11):1224–1232
Benson SC, Mathies RA, Glazer AN (1993) Heterodimeric DNA-binding dyes designed for energy transfer: stability and applications of the DNA complexes. Nucleic Acids Res 21(24):5720–5726
Biver T, De Biasi A, Secco F, Venturini M, Yarmoluk S (2005) Cyanine dyes as intercalating agents: kinetic and thermodinamic studies on the DNA/Cyan40 and DNA/CCyan2 systems. Biophys J 89:374–383
Yang XL, Wang AH-J (1999) Structural studies of atom-specific drugs on DNA. Pharmacol Ther 83:181–215
Brańa MF, Cacho M, Gradillas A, Pascual-Teresa B, Ramos A (2001) Intercalators as anticancer drugs. Curr Pharm Des 7:1745–1780
Martinez R, Chacon-Garcia L (2005) The search of DNA-intercalators as antitumoral drugs: what it worked and what did not work. Curr Med Chem 12(2):127–151
Subramanian D, Sommer FC, Muller MT (2001) ICE Bioassay. In: Osheroff N, Bjornsti M-A (eds) DNA Topoisomerase protocols. Enzymology and drugs. Humana Press, Totowa, pp 137–147
Ehlert JE, Kubbutat MHG (2001) Apoptosis and its relevance in cancer therapy. Onkologie 24:433–440
Hurley LH (2002) DNA and its associated processes as targets for cancer therapy. Nat Rev Cancer 2:188–200
Akimitsu N, Kamura K, Toné S, Sakaguchi A, Kikuchi A, Hamamoto H, Sekimizu K (2003) Induction of apoptosis by depletion of topoisomerase II in mammalian cells. Biochem Biophys Res Commun 307:301–307
Kaina B (2003) DNA damage-triggered apoptosis: critical role of DNA repair, double-strand breaks, cell proliferation and signaling. Biochem Pharmacol 66:1547–1554
Palm BS, Piantanida I, Žinić M, Schneider H-J (2000) The interaction of new 4,9-diazapyrenium compounds with double stranded nucleic acids. J Chem Soc – Perkin Trans II:385–392
Piantanida I, Tomšić V, Žinić M (2000) 4,9-Diazapyrenium cations. Synthesis, physico-chemical properties and binding of nucleotides in water. J Chem Soc Perkin Trans II:375–383
Piantanida I, Palm BS, Žinić M, Schneider H-J (2001) A new 4,9-diazapyrenium intercalator for single- and double-stranded nucleic acids: distinct differences from related diazapyrenium compounds and ethidium bromide. J Chem Soc Perkin Trans II(9):1808–1816
Yu D-H, MacDonald J, Josephs S, Liu Q, Nguy V, Tor Y, Wong-Staal F, Li Q-X (2006) MDDD, a 4,9-diazapyrenium derivative, is selectively toxic to glioma cells by inducing growth arrest at G0/G1 independently of p53. Invest New Drugs 24:489–498
Steiner-Biočić I, Glavaš-Obrovac L, Karner I, Piantanida I, Žinić M, Pavelić K, Pavelić J (1996) 4,9-Diazapyrenium dications induce apoptosis in human tumor cells. Anticancer Res 16:3705–3708
Roknić S, Glavaš-Obrovac L, Karner I, Piantanida I, Žinić M, Pavelić K (2000) In vitro cytotoxicity of three 4,9-diazapyrenium hydrogensulfate derivatives on different human tumor cell lines. Chemotherapy 46:143–149
Marczi S, Glavaš-Obrovac L, Karner I (2005) Induction of apoptosis of human tumor cells by a 4,9-diazapyrenium derivative and its effects on topoisomerase-II action. Chemotherapy 51:217–222
Piantanida I, Žinić M, Marczi S, Glavaš-Obrovac L (2007) Bis-4,9-diazapyrenium dications: synthesis of the methylenedibenzyl-analogue, interactions with nucleotides, DNA, RNA. The antitumor activity of all till now prepared analogues. J Phys Org Chem 20:285–295
Mickisch G, Fajta S, Keilhauer G, Schlick E, Tschada R, Alken P (1990) Chemosensitivity testing of primary human renal cell carcinoma by a tetrazolium based microculture assay (MTT). Urol Res 18:131–136
Burden DA, Froelich-Ammon J, Osheroff N (2001) Topoisomerase I-mediated cleavage of plasmid DNA. In: Osheroff N, Bjornsti M-A (eds) DNA topoisomerase protocols. Enzymology and drugs. Humana Press Inc., New Yersey, pp 283–289
Kaufmann SH, Svingen PA (2001) Immunoblot analysis and bend depletion assay. In: Bjornsti M-A, Osheroff N (eds), DNA topoisomerase protocols. DNA topology and enzymes. Humana Press Inc., New Yersey, pp 253–268
Laemmli UK (1970) Cleavage of structural proteins during the assembly of head of bacteriophage T4. Nature 227:680–685
Gedda L, Silvander M, Sjöberg S, Tjarks W, Carlsson J (1997) Cytotoxicity and subcellular localization of boronated phenanthridinium analogues. Anticancer Drug Des 12:671–685
Haldane A, Finlay GJ, Hay MP, Denny WA, Baguley BC (1999) Cellular uptake of N-[2-(dimethylamino)ethyl]acridine-4-carboxamide (DACA). Anticancer Drug Des 14:275–280
Hicks KO, Pruijn FB, Baguley BC, Wilson WR (2001) Extravascular transport of the DNA intercalator and topoisomerase poison N-[2-(dimethylamino)ethyl]acridine-4-carboxamide (DACA): diffusion and metabolism in multicellular layers of tumor cells. J Pharmacol Exp Ther 297:1088–1098
Lansiaux A, Dassonneville L, Facompré M, Kumar A, Stephens CE, Bajic M, Tanious F, Wilson WD, Boykin DW, Bailly C (2002) Distribution of furamidine analogues in tumor cells: influence of the number of positive charges. J Med Chem 45:1994–2002
Cline S, Osheroff N (1999) Cytosine arabinoside lesions are position-specific topoisomerase II poisons and stimulate DNA cleavage mediated by the human type II enzymes. J Biol Chem 274:29740–29743
Rowe TC, Grabowski D, Ganapathi R (2001) Isolation of covalent enzyme-DNA complexes. In: Osheroff N, Bjornsti M-A (eds) DNA topoisomerase protocols. Enzymology and drugs. Humana Press Inc, New Jersey, pp 129–136
Kim SG, Sung M, Kang KW, Kim SH, Son MH, Kim WB (2001) DA-125, a novel anthracycline derivative showing high-affinity DNA binding and topoisomerase II inhibitory activities, exerts cytotoxicity via c-Jun N-terminal kinase pathway. Cancer Chemother Pharmacol 47:511–518
Bal C, Baldeyrou B, Moz F, Lansiaux A, Colson P, Kraus-Berthier L, Leonce S, Pierre A, Boussard M-F, Rousseau A, Wierzbicki M, Bailly C (2004) Novel antitumor indenoindole derivatives targeting DNA and topoisomerase II. Biochem Pharmacol 68:1911–1922
Marsh KL, Willmore E, Tinelli S, Cornarotti M, Mectes EL, Capranico G, Fisher LM, Austin CA (1996) Amsacrine-promoted DNA cleavage site determinants for the two human DNA topoisomerase II isoforms alpha and beta. Biochem Pharmacol 52:1675–1685
Finlay GJ, Atwell GJ, Baguley BC (1999) Inhibition of the action of the topoisomerase II poison amsacrine by simple aniline derivatives: evidence for drug-protein interactions. Oncol Res 11(6):249–254
Yew N, Strobel M, Vande Woude GF (1993) Mos and the cell cycle: the molecular basis of the transformed phenotype. Curr Opin Genet Dev 3(1):19–25
Fukasawa K, Vande Woude GF (1997) Synergy between the Mos/mitogen-activated protein kinase pathway and loss of p53 function in transformation and chromosome instability. Mol Cell Biol 17(1):506–518
Lyons SK, Clarke AR (1997) Apoptosis and carcinogenesiy. Br Medical Bull 53:554–569
Djelloul S, Forgue-Lafitte M-E, Hermelin B, Mareel M, Bruyneel E, Baldi A, Giordano A, Chastre E, Gespach C (1997) Enterocyte differentiation is compatible with SV40 large T expression and loss of p53 function in human colonic Caco-2 cells. FEBS Lett 406:234–242
Pietsch EC, Humbey O, Murphy ME (2006) Polymorphisms in the p53 pathway. Oncogene 25:1602–1611
Thompson CB (1995) Apoptosis in the pathogenesis and treatment of disease. Science 267:1456–1462
Mayo MW, Wang CY, Cogswell PC, Rogers-Graham KS, Lowe SW, Der CJ, Baldwin AS Jr (1997) Requirement of NF-κB activation to suppress p53-independent apoptosis induced by oncogenic Ras. Science 278:1812–1815
Shapiro P (2001) Ras-MAP kinase signaling pathways and control of cell proliferation: relevance to cancer therapy. Crit Rev Clin Lab Sci 39(4–5):285–330
Moon A (2006) Differential functions of Ras for malignant phenotypic conversion. Arch Pharm Res 29(2):113–122
Ward RL, Todd AV, Santiago F, O’Connor T, Hawkins NJ (1997) Activation of the K-ras oncogene in colorectal neoplasms is associated with decreased apoptosis. Cancer 79(6):1106–1113
Downward J (1998) ras signaling and apoptosis. Curr Opin Genet Dev 8:49–54
Bowen C, Voeller HJ, Kikly K, Gelmann EP (1999) Synthesis of procaspase-3 and -7 during apoptosis in prostate cancer cells. Cell Death Differ 6:394–401
Moore DM (2000) Hematology of the mouse. In: Feldman BV, Zinkl JG, Jain NC (eds) Schalm’s veterinary hematology. Lippincot Williams & Wilkons, Philadelphia, pp 1219–1224
Acknowledgments
This project was supported by the Ministry of Science, Education and Sport, Republic of Croatia, grants 0127111 and 219-0982914-2176. We are grateful to Prof. Dr. Mirjana Knežević-Kostović and M.Sc. Neda Cetina for their kind support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Marczi, S., Glavaš-Obrovac, L., Belovari, T. et al. Biological properties of 4-methyl-2,7-diamino-5,10-diphenyl-4,9-diazapyrenium hydrogensulfate (ADAP). Cancer Chemother Pharmacol 62, 595–604 (2008). https://doi.org/10.1007/s00280-007-0643-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-007-0643-0