Abstract
Purpose
Irinotecan, a topoisomerase I inhibitor, is an effective agent for non-small-cell lung cancer (NSCLC). To determine the efficacy and toxicity of irinotecan and carboplatin, we conducted a phase II study in 61 patients with advanced NSCLC.
Methods
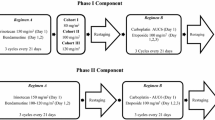
Every 4 weeks, the patients received irinotecan 50 mg/m2 (days 1, 8 and 15) and carboplatin (day 1) with a target AUC of 5 mg min/ml using the Chatelut formula.
Results
All patients were evaluable for toxicity, and of 59 patients evaluable for response, 20 achieved a partial response and 26 showed no change. The overall response rate was 34% (95% confidence interval 23–48%). Grade 3 or 4 anemia, leukopenia, neutropenia, thrombocytopenia and diarrhea occurred in 32%, 32%, 60%, 25%, and 7%, respectively. The median survival time and 1-year, and 2-year survival rates were 10.0 months, 37.6%, and 15.2%, respectively.
Conclusions
Irinotecan with carboplatin is effective for advanced NSCLC and safe.

Similar content being viewed by others
References
Hsiang YH, Liu LF (1988) Identification of mammalian DNA topoisomerase I as an intracellular target of the anticancer drug camptothecin. Cancer Res 48:1722
Hertzberg RP, Caranfa MJ, Holden KG, Jakas DR, Gallagher G, Mattern MR, Mong SM, Bartus JO, Johnson RK, Kingsbury WD (1989) Modification of the hydroxyl lactone ring of camptothecin: inhibition of mammalian topoisomerase I and biological activity. J Med Chem 32:715
Masuda N, Kudoh S, Fukuoka M (1996) Irinotecan (CPT-11): pharmacology and clinical applications. Crit Rev Oncol Hematol 24:3
Fukuda M, Nishio K, Kanzawa F, Ogasawara H, Ishida T, Arioka H, Bojanowski K, Oka M, Saijo N (1996) Synergism between cisplatin and topoisomerase I inhibitors, NB-506 and SN-38, in a human small cell lung cancer cells. Cancer Res 56:789
Kano Y, Akutsu M, Suzuki K, Yoshida M (1993) Effects of carboplatin in combination with other anticancer agents on human leukemia cell lines. Leuk Res 17:113
Kanzawa F, Sugimoto Y, Minato K, Kasahara K, Bungo M, Nakagawa K, Fujiwara Y, Liu LF, Saijo N (1990) Establishment of a camptothecin analogue (CPT-11)-resistant cell line of human non-small cell lung cancer: characterization and mechanism of resistance. Cancer Res 50:5919
Masuda N, Fukuoka M, Fujita A, Kurita Y, Tsuchiya S, Nagao K, Negoro S, Nishikawa H, Katakami N, Nakagawa K, Niitani H (1998) Phase II trial of combination of CPT-11 and cisplatin for advanced non-small-cell lung cancer. Br J Cancer 78:251
Negoro S, Masuda N, Takada Y, Sugiura T, Kudoh S, Katakami N, Ariyoshi Y, Ohashi Y, Niitani H, Fukuoka M, CPT-11 Lung Cancer Study Group West (2003) Randomized phase III trial of irinotecan combined with cisplatin for advanced non-small-cell lung cancer. Br J Cancer 88:335
Niho S, Nagao K, Nishiwaki Y, Yokoyama A, Saijo N, Ohashi Y, Niitani H, The Irinotecan Lung Cancer Study Group (1999) Randomized multicenter phase III trial of irinotecan (CPT-11) and cisplatin (CDDP) versus CDDP and vindesine (VDS) in patients with advanced non-small cell lung cancer (NSCLC). Proc Am Soc Clin Oncol 17:492a
De Vore RF, Johnson DH, Crawford J, Garst J, Dimery IW, Eckardt J, Eckhardt SG, Elfring GL, Schaaf LJ, Hanover CK, Miller LL (1999) Phase II study of irinotecan plus cisplatin in patients with advanced non-small-cell lung cancer. J Clin Oncol 17:2710
Rose WC, Schurig JE (1985) Preclinical antitumor and toxicologic profile of carboplatin. Cancer Treat Rev 12:1
Lokich J, Anderson N (1998) Carboplatin versus cisplatin in solid tumors: an analysis of the literature. Ann Oncol 9:13
Bunn PA Jr (2002) Chemotherapy for advanced non-small-cell lung cancer: who, what, when, why? J Clin Oncol 20:23S
Fukuda M, Oka M, Soda H, Terashi K, Kawabata S, Nakatomi K, Takatani H, Tsurutani J, Tsukamoto K, Noguchi Y, Fukuda M, Kinoshita A, Kohno S (1999) Phase I study of irinotecan combined with carboplatin in previously untreated solid cancers. Clin Cancer Res 5:3936
Calvert AH, Newell DR, Gumbrell LA, O’Reilly S, Burnell M, Boxall FE, Siddik ZH, Judson IR, Gore ME, Wiltshaw E (1989) Carboplatin dosage: prospective evaluation of a simple formula based on renal function. J Clin Oncol 7:1748
Egorin MJ, Van Echo DA, Olman EA, Whitacre MY, Forrest A, Aisner J (1985) Prospective validation of a pharmacologically based dosing schema for the cis-diamminedichloroplatinum (II) analogue diamminecyclobutanedicarboxylato-platinum. Cancer Res 45:6502
Duffull SB, Robinso BA (1997) Clinical pharmacokinetics and dose optimisation of carboplatin. Clin Pharmacokinet 33:161
Chatelut E, Canal P, Brunner V, Chevreau C, Pujol A, Boneu A, Roche H, Houin G, Bugat R (1995) Prediction of carboplatin clearance from standard morphological and biological patient characteristics. J Natl Cancer Inst 87:573
Mountain CF, Dresler CM (1997) Regional lymph node classification for lung cancer staging. Chest 111:1718
WHO (1979) Handbook for reporting results of cancer treatment. World Health Organization, Geneva
Simon R (1987) How large should be a phase II trial of a new drug? Cancer Treat Rep 71:1079
Kaplan E, Meier P (1958) Nonparametric estimation from incomplete observations. J Am Stat Assoc 53:457
Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, Zhu J, Johnson DH, Eastern Cooperative Oncology Group (2002) Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med 346:92
Takeda K, Negoro S, Ohashi Y, Saijo N, Nishiwaki Y, Tamura T, Nakagawa K, Kubota K, Ariyoshi Y, Fukuoka M (2003) Preliminary results of four arm cooperative study (FACS) for advanced non-small cell lung cancer (NSCLC) in Japan. Lung Cancer 41:S64
Takeda K, Takefuji N, Uejima H, Yoshimura N, Terakawa K, Negoro S (2002) Phase II study of irinotecan and carboplatin for advanced non-small cell lung cancer. Lung Cancer 38:303
Kelly K, Crowley J, Bunn PA Jr, Presant CA, Grevstad PK, Moinpour CM, Ramsey SD, Wozniak AJ, Weiss GR, Moore DF, Israel VK, Livingston RB, Gandara DR (2001) Randomized phase III trial of paclitaxel plus carboplatin versus vinorelbine plus cisplatin in the treatment of patients with advanced non-small-cell lung cancer: a Southwest Oncology Group trial. J Clin Oncol 19:3210
Author information
Authors and Affiliations
Consortia
Corresponding author
Rights and permissions
About this article
Cite this article
Fukuda, M., Oka, M., Soda, H. et al. Phase II study of irinotecan combined with carboplatin in previously untreated non-small-cell lung cancer. Cancer Chemother Pharmacol 54, 573–577 (2004). https://doi.org/10.1007/s00280-004-0805-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-004-0805-2