Abstract
Purpose
To develop a population pharmacokinetic model of vinorelbine administered by short intravenous infusion in metastatic breast cancer patients.
Methods
Vinorelbine was administered as infusions of 5–10 min at 15, 20 or 25 mg/m2 to 30 patients. Blood samples were collected over 18 h. Plasma concentrations of vinorelbine were determined by HPLC. Population pharmacokinetic analysis was performed using a nonlinear mixed effects modeling method.
Results
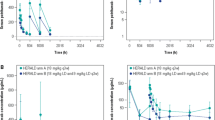
Vinorelbine concentration-time profiles were best described by a three-compartment open model. Plasma clearance (CL) was high and positively related to lean body weight (LBW) and body surface area (BSA) or to a combination of height and body weight (BW). Elevated serum alkaline phosphatases had a negative effect on CL. Typical population estimates of CL and central distribution volume (V1) were 74.2 l/h and 7.8 l, respectively. The interindividual population coefficients of variation for CL and V1 were 17.0% and 32.0%, respectively. The stability and predictive performance of the final population pharmacokinetic model were assessed using 200 bootstrap samples of the original data.
Conclusion
This study identified combined effects of BSA and serum alkaline phosphatases on clearance. These results partly support the conventional dose adjustment of vinorelbine based on BSA, but suggest dose modification in cases of extreme values of serum alkaline phosphatases.



Similar content being viewed by others
Abbreviations
- ALT:
-
Alanine amino transferase
- AST:
-
Aspartate amino transferase
- AUC:
-
Area under the concentration curve
- BSA:
-
Body surface area
- BW:
-
Body weight
- CL:
-
Clearance
- ISV:
-
Intersubject variability
- LBW:
-
Lean body weight
- OFV:
-
Objective function value
- SAP:
-
Serum alkaline phosphatases
- SCr:
-
Serum creatinine
- V1 :
-
Distribution volume
References
Chatelut E, Boddy AV, Peng B, Rubie H, Lavit M, Dezeuze A, Pearson AD, Roche H, Robert A, Newell DR, Canal P (1996) Population pharmacokinetics of carboplatin in children. Clin Pharmacol Ther 59:436
Freyer G, Tranchand B, Ligneau B, Ardiet C, Souquet PJ, Court-Fortune I, Riou R, Rebattu P, Boissel JP, Trillet-Lenoir V, Girard P (2000) Population pharmacokinetics of doxorubicin, etoposide and ifosfamide in small cell lung cancer patients: results of a multicentre study. Br J Clin Pharmacol 50:315
Gallo JM, Laub PB, Rowinsky EK, Grochow LB, Baker SD (2000) Population pharmacokinetic model for topotecan derived from phase I clinical trials. J Clin Oncol 18:2459
Gauvin A, Pinguet F, Culine S, Astre C, Gomeni R, Bressolle F (2000) Bayesian estimate of vinorelbine pharmacokinetic parameters in elderly patients with advanced metastatic cancer. Clin Cancer Res 6:2690
Gauvin A, Pinguet F, Culine S, Astre C, Cupissol D, Bressolle F (2002) Blood and plasma pharmacokinetics of vinorelbine in elderly patients with advanced metastatic cancer. Cancer Chemother Pharmacol 49:48
Gauvin A, Pinguet F, Culine S, Astre C, Gomeni R, Bressolle F (2002) A limited-sampling strategy to estimate individual pharmacokinetic parameters of vinorelbine in elderly patients with advanced metastatic cancer. Anticancer Drugs 13:473
Gurney HP, Ackland S, Gebski V, Farrell G (1998) Factors affecting epirubicin pharmacokinetics and toxicity: evidence against using body-surface area for dose calculation. J Clin Oncol 16:2299
Khayat D, Covelli A, Variol P (1995) Phase I and pharmacologic study of intravenous vinorelbine in patients with solid tumors. Proc Am Soc Clin Oncol 14:371
Marquet P, Lachatre G, Debord J, Eichler B, Bonnaud F, Nicot G (1992) Pharmacokinetics of vinorelbine in man. Eur J Clin Pharmacol 42:545
Mouly S, Aymard G, Tillement JP, Caulin C, Bergmann JF, Urien S (2001) Increased oral gancyclovir bioavailability in HIV-infected patients with chronic diarrhea and wasting syndrome—a population pharmacokinetic study. Br J Clin Pharmacol 51:557
Nguyen L, Tranchand B, Puozzo C, Variol P (2002) Population pharmacokinetics model and limited sampling strategy for intravenous vinorelbine derived from phase I clinical trials. Br J Clin Pharmacol 53:459
Parke J, Charles BG (2000) Factors affecting oral cyclosporin disposition after heart transplantation: bootstrap validation of a population pharmacokinetic model. Eur J Clin Pharmacol 56:481
Sabot C, Marquet P, Debord J, Carpentier N, Merle L, Lachatre G (1998) Bayesian pharmacokinetic estimation of vinorelbine in non-small-cell lung cancer patients. Eur J Clin Pharmacol 54:171
Schilling T, Fiebig HH, Kerpel-Fronius S, Winterhalter B, Variol P, Tresca P, Heinrich B, Hanauske AR (1996) Clinical phase I and pharmacokinetic trial of vinorelbine administered as single intravenous bolus every 21 days in cancer patients. Invest New Drugs 14:371
Urien S (1997) MP2 (V 2.0)—a Windows application for population pharmacokinetics. In: Aarons L, Balant LP, Danhof M, et al (eds) The population approach: measuring and managing variability in response, concentration. European Community, Brussels, p 419
Urien S, Bree F, Breillout F, Bastian G, Krikorian A, Tillement JP (1993) Vinorelbine high-affinity binding to human platelets and lymphocytes: distribution in human blood. Cancer Chemother Pharmacol 32:231
Urien S, Fumoleau P, Campone M, Kerbrat P, Bonneterre J, Fargeot P, Deporte-Fety R (2003) Modelling of ftorafur and 5-fluorouracil pharmacokinetics following oral UFT administration. A population study in 30 patients with advanced breast cancer. Cancer Chemother Pharmacol 52:99
Variol P, Nguyen L, Tranchand B, Puozzo C (2002) A simultaneous oral/intravenous population pharmacokinetic model for vinorelbine. Eur J Clin Pharmacol 58:467
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Deporte-Fety, R., Simon, N., Fumoleau, P. et al. Population pharmacokinetics of short intravenous vinorelbine infusions in patients with metastatic breast cancer. Cancer Chemother Pharmacol 53, 233–238 (2004). https://doi.org/10.1007/s00280-003-0729-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-003-0729-2