Abstract
Purpose
The purpose of this analysis was to develop a population pharmacokinetic (PK) model for patritumab, a fully human monoclonal antibody that targets human epidermal growth factor receptor 3.
Methods
A total of 833 serum concentrations were included in this analysis; serum concentrations were obtained from 145 subjects (136 with non-small cell lung cancer, nine with solid tumors) treated with patritumab [9 or 18 mg/kg intravenously every 3 weeks (q3w)] in one phase 1 and one phase 1b/2 study. Data were analyzed by nonlinear mixed-effect modeling.
Results
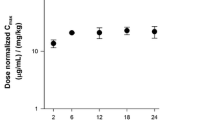
Patritumab PKs were best described through a two-compartment model with first-order elimination and interindividual variability on clearance (CL), volume of the central compartment (V c), distributional clearance, and volume of the peripheral compartment. In the final model, CL and V c were estimated as 0.0238 L/h and 3.62 L, respectively. Body weight (BW) and baseline albumin were found to be covariates for CL and BW was a covariate for V c. Covariates associated with hepatic and renal impairment were not significant on CL. Simulations showed that BW-based dosing reduced interindividual variability in patritumab exposure compared with fixed dosing.
Conclusions
The PK of patritumab was linear at the doses studied and well described by the two-compartment model. Hepatic and renal impairment did not appear to affect PK. Our results support BW-based dosing of patritumab on a q3w schedule.




Similar content being viewed by others
References
Hsieh AC, Moasser MM (2007) Targeting HER proteins in cancer therapy and the role of the non-target HER3. Br J Cancer 97:453–457
Liu J, Kern JA (2002) Neuregulin-1 activates the JAK–STAT pathway and regulates lung epithelial cell proliferation. Am J Respir Cell Mol Biol 27:306–313
Hegde GV, de la Cruz CC, Chiu C, Alag N, Schaefer G, Crocker L, Ross S, Goldenberg D, Merchant M, Tien J, Shao L, Roth L, Tsai SP, Stawicki S, Jin Z, Wyatt SK, Carano RA, Zheng Y, Sweet-Cordero EA, Wu Y, Jackson EL (2013) Blocking NRG1 and other ligand-mediated Her4 signaling enhances the magnitude and duration of the chemotherapeutic response of non-small cell lung cancer. Sci Transl Med 5:171ra18
Bièche I, Onody P, Tozlu S, Driouch K, Vidaud M, Lidereau R (2003) Prognostic value of ERBB family mRNA expression in breast carcinomas. Int J Cancer 106:758–765
Yi ES, Harclerode D, Gondo M, Stephenson M, Brown RW, Younes M, Cagle PT (1997) High c-erbB-3 protein expression is associated with shorter survival in advanced non-small cell lung carcinomas. Mod Pathol 10:142–148
Shames DS, Carbon J, Walter K, Jubb AM, Kozlowski C, Januario T, AnDo FuL, Xiao Y, Raja R, Jiang B, Malekafzali A, Stern H, Settleman J, Wilson TR, Hampton GM, Yauch RL, Pirzkall A, Amler LC (2013) High heregulin expression is associated with activated HER3 and may define an actionable biomarker in patients with squamous cell carcinomas of the head and neck. PLoS ONE 8:e56765
Xia W, Gerard CM, Liu L, Baudson NM, Ory TL, Spector NL (2005) Combining lapatinib (GW572016), a small molecule inhibitor of ErbB1 and ErbB2 tyrosine kinases, with therapeutic anti-ErbB2 antibodies enhances apoptosis of ErbB2-overexpressing breast cancer cells. Oncogene 24:6213–6221
Baselga J, Swain SM (2009) Novel anticancer targets: revisiting ERBB2 and discovering ERBB3. Nat Rev Cancer 9:463–475
Garrett JT, Olivares MG, Rinehart C, Granja-Ingram ND, Sánchez V, Chakrabarty A, Dave B, Cook RS, Pao W, McKinely E, Manning HC, Chang J, Arteaga CL (2011) Transcriptional and posttranslational up-regulation of HER3 (ErbB3) compensates for inhibition of the HER2 tyrosine kinase. Proc Natl Acad Sci USA 108:5021–5026
Sergina NV, Rausch M, Wang D, Blair J, Hann B, Shokat KM, Moasser MM (2007) Escape from HER-family tyrosine kinase inhibitor therapy by the kinase-inactive HER3. Nature 445:437–441
Freeman D, Ogbagabriel S, Rothe M, Radinsky R, Treder M (2008) Fully human anti-HER3 monoclonal antibodies (mAbs) have unique in vitro and in vivo functional and antitumor activities versus other HER family inhibitors. In: Abstract presented at 99th AACR annual meeting, San Diego, CA, 12–16 April 2008 (Abstract LB-21)
Treder M, Hartmann S, Ogbagabriel S, Borges E, Green L, Kang J, Radinsky R, Rothe M, Freeman D (2008) Fully human Anti-HER3 monoclonal antibodies (mAbs) inhibit oncogenic signaling and tumor cell growth in vitro and in vivo. In: Abstract presented at 99th AACR annual meeting, San Diego, CA, 12–16 April 2008 (Abstract LB-20)
Treder M, Ogbagabriel S, Moor R, Schulze-Horsel U, Hettmann T, Rothe M, Radinsky R, Freeman D (2008) Fully human anti-HER3 mAb U3-1287 (AMG 888) demonstrates unique in vitro and in vivo activities versus other HER family inhibitors in NSCLC models. Eur J Cancer 6(suppl):99 (Abstract 309)
LoRusso P, Jänne PA, Oliveira M, Rizvi N, Malburg L, Keedy V, Yee L, Copigneaux C, Hettmann T, Wu CY, Ang A, Halim AB, Beckman RA, Beaupre D, Berlin J (2013) Phase I study of U3-1287, a fully human anti-HER3 monoclonal antibody, in patients with advanced solid tumors. Clin Cancer Res 19:3078–3087
Wakui H, Yamamoto N, Nakamichi S, Tamura Y, Nokihara H, Yamada Y, Tamura T (2014) Phase 1 and dose-finding study of patritumab (U3-1287), a human monoclonal antibody targeting HER3, in Japanese patients with advanced solid tumors. Cancer Chemother Pharmacol 73:511–516
Von Pawel J, Tseng J, Dediu M, Schumann C, Moritz B, Mendell-Harary J, Jin X, Feng W, Copigneaux C, Beckman RA (2014) Phase 2 HERALD study of patritumab (P) with erlotinib (E) in advanced NSCLC subjects (SBJs). J Clin Oncol 32(15 suppl):8045
Dirks NL, Meibohm B (2010) Population pharmacokinetics of therapeutic monoclonal antibodies. Clin Pharmacokinet 49:633–659
Wang W, Wang EQ, Balthasar JP (2008) Monoclonal antibody pharmacokinetics and pharmacodynamics. Clin Pharmacol Ther 84:548–558
Lum P, Perez Ruixo JJ, Ogbagabriel S, DoshI S, Chen A, Yang BB, Hettman T, Radinsky R, Freeman D (2009) Identifying first in human (FIH) doses and schedule of U3-1287 (AMG 888), a fully human anti-HER3 mAb, based on preclinical pharmacokinetic (PK), pharmacodynamic (PD) and efficacy data. Mol Cancer Ther 8(12 suppl):B167. doi:10.1158/1535-7163.TARG-09-B167
Cockcroft DW, Gault MH (1976) Prediction of creatinine clearance from serum creatinine. Nephron 16:31–41
Stevens LA, Coresh J, Greene T, Levey AS (2006) Assessing kidney function-measured and estimated glomerular filtration rate. N Engl J Med 354:2473–2483
Yang J, Shord S, Zhao H, Men Y, Rahman A (2013) Are hepatic impairment studies necessary for therapeutic proteins? Clin Ther 35:1444–1450
Xu Z, Vu T, Lee H, Hu C, Ling J, Yan H, Baker D, Beutler A, Pendley C, Wagner C, Davis HM, Zhou H (2009) Population pharmacokinetics of golimumab, an antitumor necrosis factor-alpha human monoclonal antibody, in patients with psoriatic arthritis. J Clin Pharmacol 49:1056–1070
Mager DE, Jusko WJ (2001) General pharmacokinetic model for drugs exhibiting target-mediated drug disposition. J Pharmacokinet Pharmacodyn 28:507–532
Bai S, Jorga K, Xin Y, Jin D, Zheng Y, Damico-Beyer LA, Gupta M, Tang M, Allison DE, Lu D, Zhang Y, Joshi A, Dresser MJ (2012) A guide to rational dosing of monoclonal antibodies. Clin Pharmacokinet 51:119–135
Paz-Ares L, Pawel Jv, Moritz B, Mendell J, Jin X, Copigneaux C, Beckman R (2014) Phase (Ph) 3 study of patritumab (P) plus erlotinib (E) in EGFR wild-type subjects with advanced non–small cell lung cancer (NSCLC). In: Poster presented at annual meeting of the European Society of Medical Oncology, Madrid, Spain, 26–30 September 2014 (Abstract 1336TiP)
Acknowledgments
Third-party writing assistance was provided by BlueMomentum, an Ashfield Company, part of UDG Healthcare plc, and supported by Daiichi Sankyo, Inc. and Daiichi Sankyo Co., Ltd.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Satoshi Yoshiba, Mendel Jansen, Shuquan Chen, and Jeanne Mendell are employees of Daiichi Sankyo. Nobuko Matsushima is a former employee of Daiichi Sankyo.
Ethical standard
The data used in this analysis were collected from two studies that were approved by the institutional review board of participating institutions and conducted in accordance with the principles of the Declaration of Helsinki.
Rights and permissions
About this article
Cite this article
Yoshiba, S., Jansen, M., Matsushima, N. et al. Population pharmacokinetic analysis of patritumab, a HER3 inhibitor, in subjects with advanced non-small cell lung cancer (NSCLC) or solid tumors. Cancer Chemother Pharmacol 77, 987–996 (2016). https://doi.org/10.1007/s00280-016-3011-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-016-3011-0