Abstract Purpose:
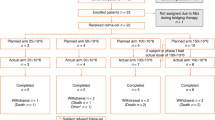
The current study was initiated to assess the clinical efficacy and side effects of rituximab in patients with relapsed advanced stage follicular lymphoma. Patients and methods: The study was performed as an open-label non-randomized multicenter phase-II trial and included patients older than 18 years of age with relapsed advanced-stage follicular lymphomas (FL) grades I and II, according to the REAL classification, or with centroblastic/centrocytic (CB/CC lymphomas according to the Kiel classification. Four weekly doses of 375 mg/m2 rituximab were applied. Results: 38 patients from eight centers were included between January 1997 and January 1998 and were evaluable for response and toxicity on an intention to treat basis. The median age was 55 years (range 26–75 years). Thirteen patients (35%) were in first relapse, 11 patients (30%) in second, and 13 patients (35%) in third relapse. The median time between primary diagnosis and study entry was 4.6 years (range 0.9–14.7 years). Twenty-three patients tolerated the application of rituximab without adverse events; in 13 cases the infusion rate had to be reduced because of side effects; in two patients the application was stopped because of pharyngeal edema and anaphylactoid reaction. The most frequent side effects were fever (13 patients) and rigor (13 patients); 65% of the side effects were observed after the first infusion. Twenty grade-III/IV side effects were considered to be related to treatment: lymphocytopenia (3), granalocytopenia (1), thrombocytopenia (2), fever (1), hyperglycermia (1), venous thrombosis (1), syncope (1), plasmatic coagulation disorder (1), shortness of breath (2), photosensitivity (1), cardiac failure (1), chills (1), sepsis (1), tumor lysis (1), anemia (1), and pharyngeal edema (1). Eight patients were not eligible for assessment of response because of non-follicular subtypes of low-grade lymphomas (n=6) or early termination of therapy at the first infusion because of severe side effects (n=2). From the 30 evaluable cases with follicular lymphomas, five patients achieved a complete remission (CR) (17%), nine patients a partial remission (PR) (30%), and two patients a minor response (MR) (7%). The overall response rate was 47%. The median time to treatment progression (TTP) was 201 days (range 64–293 days), with five patients experiencing long-lasting remissions of 214–293 days duration. In three patients, the rituximab-induced remission exceeded the preceding progression-free interval substantially. Bulky disease (P=0.058) and/or bone-marrow involvement (P=0.046) were associated with poor response. Conclusion: This study confirms the moderate treatment-related toxicity and the high antilymphoma activity of rituximab in patients with relapsed follicular lymphoma. Further studies are needed to determine the role of rituximab in the first-line treatment of these disorders and its combination with conventional chemotherapy.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 29 September 1999 / Accepted: 23 December 1999
Rights and permissions
About this article
Cite this article
Feuring-Buske, M., Kneba, M., Unterhalt, M. et al. IDEC-C2B8 (Rituximab) anti-CD20 antibody treatment in relapsed advanced-stage follicular lymphomas: results of a phase-II study of the German Low-Grade Lymphoma Study Group. Ann Hematol 79, 493–500 (2000). https://doi.org/10.1007/s002770000163
Issue Date:
DOI: https://doi.org/10.1007/s002770000163