Abstract
Secondary central nervous system involvement (sCNSi) in diffuse large B-cell lymphoma (DLBCL) is fatal. However, its features in patients with sCNSi who are categorized as lower risk by international prognostic index (IPI) or CNS-IPI are not yet fully understood. In the present analysis, we evaluated DLBCL patients who developed sCNSi at their first progression and who participated in JCOG0601, most of whom were lower risk by IPI. Of 409 patients, 21 (5.1%) developed sCNSi during a median follow-up of 4.9 years. Five-year cumulative incidence of sCNSi were 5.1%; and 4.0%, 5.3%, and 11.5% at low, intermediate, and high risk of CNS-IPI, respectively. The most common locations of extranodal lesions at the time of registration in patients with sCNSi were the stomach (n = 4), paranasal cavity (n = 3), and bone marrow (n = 2). In univariable analysis, paranasal cavity lesion was a high-risk factor for sCNSi (subdistribution hazard ratio, 4.34 [95% confidence interval 1.28–14.73]). Median overall survival after sCNSi was 1.3 years, with a 2-year overall survival rate of 39.3%. The incidence of sCNSi in DLBCL patients at lower risk of CNS-IPI was low, as previously reported, but paranasal cavity lesion might indicate high risk for organ involvement.
Clinical trial registration
JCOG0601 was registered in the UMIN Clinical Trials Registry (UMIN000000929, date of registration; December 04, 2007) and the Japan Registry of Clinical Trials (jRCTs031180139, date of registration; February 20, 2019).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Secondary central nervous system involvement (sCNSi) develops in approximately 5% of patients with diffuse large B-cell lymphoma (DLBCL) [1,2,3,4,5]. However, the risk is as high as 40% in certain high-risk patients, such as those harboring MYC/BCL2 dual translocation with or without BCL6 translocation or MYC/BCL2 dual expression, and in those with kidney and/or adrenal gland involvement [6,7,8]. CNS prophylaxis is applied to these high-risk patients in clinical practice; however, the role of CNS prophylaxis in DLBCL is currently under debate [9,10,11,12,13].
Despite the limited overall risk of sCNSi in DLBCL patients, attention is paid to sCNSi for the reason that the prognosis is extremely poor, with a median overall survival (OS) after the development of sCNSi of only a few months [9]. Among the many efforts to avoid this complication, a representative solution to prevent sCNSi in a specific subtype of DLBCL is that for primary testicular lymphoma. Regarding the high risk of sCNSi even in limited-stage primary testicular lymphoma patients, as reported in a retrospective analysis, the application of intrathecal chemotherapy and contralateral testicular irradiation displayed a dramatic reduction of the risk in a phase 2 trial [14, 15]. For intravascular large B-cell lymphoma, a peculiar subtype of DLBCL with high risk of sCNSi, standard immunochemotherapy combined with CNS-directed therapy including high-dose methotrexate and intrathecal chemotherapy also revealed better outcomes in a recent phase 2 trial than in historical controls [16, 17]. The success shown in these specific subtypes implies that an appropriate approach would be useful for reducing the risk of sCNSi; however, among patients considered to be lower risk according to currently used predictive indexes, the characteristics of those who developed sCNSi are largely unknown.
JCOG0601 is a randomized phase 2/3 trial that compared standard R-CHOP-21 with RW-CHOP-21, in which rituximab was administered weekly 8 times from the commencement of treatment in untreated DLBCL patients without CNS involvement [18]. Most of the patients participating in JCOG0601 were categorized as lower risk by the international prognostic index (IPI), which means that most patients were also in the lower risk category of CNS-IPI [19, 20]. The primary analysis revealed that RW-CHOP was not superior to standard R-CHOP. As no CNS-directed therapy was allowed in JCOG0601, the JCOG0601 cohort is therefore suitable for investigation of subsequent CNS events. The present analysis was thus conducted to elucidate the characteristics of sCNSi in patients mainly at lower risk of sCNSi according to current predictive indices.
Patients and Methods
JCOG0601
JCOG0601 (jRCTs031180139) was a phase 2/3 study conducted by the Lymphoma Study Group of Japan Clinical Oncology Group (JCOG-LSG). The study compared standard R-CHOP-21 (arm A) with RW-CHOP-21 (arm B), in which rituximab (375 mg/m2) was administered weekly 8 times from the commencement of treatment. The primary endpoint of the phase 2 part was the investigator-assessed complete response (CR) rate in arm B, and that of the phase 3 part was progression-free survival (PFS) with secondary endpoints including OS and adverse events. At the beginning of the study, only DLBCL patients without CNS involvement with advanced stage and a lower IPI risk were eligible, and the protocol was amended 33 months later to permit the enrollment of patients with any IPI risk and any clinical stage because of poor accrual. No CNS-directed therapy was allowed in the study treatment. Of the 423 patients enrolled in the JCOG0601, 409 patients were eligible, and these were analyzed in the present study.
Outcomes and statistical methods
Patients who developed CNS involvement at their first progression or relapse were considered to have sCNSi. Data regarding the site of disease at the time of registration to JCOG0601 and at the time of progression or relapse were collected from case report forms. The site of disease was determined by investigators at each institution, and a central review of the radiological/cytological findings was not performed. Information regarding parenchymal or meningeal relapse was not strictly collected. Central monitoring was performed to ensure that the study was being carried out properly. Histological diagnosis before enrollment in the study was centrally reviewed by the study-specific pathologist panel. OS after progression was defined as the date from the first progression or relapse to any cause of death.
The cumulative incidence function of sCNSi was estimated with consideration of competing risks of death and non-CNS progression or relapse. The cumulative incidence function was compared among CNS-IPI groups (high vs. intermediate vs. low) and between treatment arms (arm A vs. arm B) by Gray’s test. OS after progression were estimated by the Kaplan–Meier method and compared between the sCNSi group and the non-CNSi progression or relapse group by log-rank test. In univariable analysis, subdistribution hazard ratio (sHR) was estimated by the Fine–Gray mode to assess the effect on the incidence of sCNSi of factors including age, sex, performance status, lactate dehydrogenase, clinical stage, extranodal sites, the presence of “B” symptoms, cell of origin determined by Hans’ criteria, and the sites of disease at registration (orbital cavity, paranasal cavity, bone marrow, breast, bone, kidney, adrenal gland, and testis). All statistical analyses were performed using SAS ver. 9.4.
Results
Patients
Table 1 lists the patient characteristics. In the 409 patients, the median age of both arms was 62 years, and 222 (54%) patients were older than 60 years. Two hundred twenty-seven (56%) patients were male and 335 (82%) patients were categorized as lower IPI risk. According to CNS-IPI, there were 203 (50%), 180 (44%), and 26 (6%) patients in the low, intermediate, and high risk groups, respectively. More than one extranodal lesion was observed in 43 (11%) patients, and 31 (8%) patients had bone marrow involvement. In terms of organs indicated as high risk for sCNSi by CNS-IPI, 5 (1%) patients and 9 (2%) patients had disease in kidney and adrenal gland, respectively.
Secondary CNS involvement
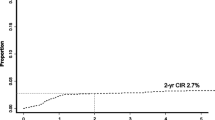
Within a median follow-up duration of 4.9 years, 21 (5%) patients developed sCNSi. The 5-year cumulative incidence of sCNSi was 5.1% (95% confidence interval [CI] 3.2–7.6), and that in patients at low, intermediate, and high risk by CNS-IPI was 4.0% (95% CI 1.9–7.4), 5.3% (95% CI 2.6–9.4), and 11.5% (95% CI 2.8–27.1), respectively (Fig. 1 a and b). Of the 21 patients, 17 (81%) developed isolated CNS involvement and the remaining 4 (19%) patients had systemic lesions other than CNS involvement simultaneously. In terms of treatment arm, the 5-year cumulative incidence was 7.3% (95% CI 4.1–11.6) in arm A and 2.9% (95% CI 1.2–5.9) in arm B (Fig. 2 a). In patients in arm A, the 5-year cumulative incidence by CNS-IPI was 5.6% (95% CI 2.1–11.8), 6.8% (95% CI 2.7–13.6), and 17.6% (95% CI 4.1–39.1) in patients at low, intermediate, and high risk, respectively (Fig. 2 b). In patients in arm B, these values were 2.7% (95% CI 0.7–7.0) patients at low risk and 3.6% (95% CI 0.9–9.2) in those at intermediate risk (Fig. 2 c). None of the high-risk patients in arm B had sCNSi. Table 2 lists the characteristics of the 21 patients with sCNSi. The CNS-IPI of these patients was low in 8, intermediate in 10, and high in 3; and 15/21 (71%) patients had extranodal involvement at the time of registration. Extranodal lesion locations found in two or more patients were the stomach (n = 4), paranasal cavity (n = 3), and bone marrow (n = 2). Of the 21 patients, the initial treatment response was progressive disease (PD) in 6 patients, and all but one patient had extranodal involvement. Five patients developed sCNSi at more than 2 years after registration, and three of these five patients did not have any extranodal lesion at the time of registration. Five of eight patients who developed sCNSi despite being low risk by CNS-IPI had extranodal involvement (stomach [n = 1], paranasal cavity [n = 1], nasal cavity [n = 1], thyroid Fn = 1], and breast [n = 1]). The median time to the development of sCNSi was 1 year (range 0.2–5.5 years). That classified by CNS-IPI was 1.3 years (range 0.3–3.6) in low, 1.1 years (range 0.3–5.5) in intermediate, and 0.5 years (range 0.2–0.9) in high-risk patients.
Prognostic factor analysis
We next analyzed prognostic factors (Table S1). In univariable analysis, paranasal cavity lesion (sHR 4.34, 95% CI 1.28–14.73, p = 0.02), orbital cavity lesion (sHR 14.16, 95% CI 1.61–124.34, p = 0.02), and age > 60 years (sHR 2.79, 95% CI 1.02–7.65 p = 0.046) were high-risk factors for sCNSi. The 2-year cumulative incidence in patients with and without paranasal cavity involvement was 18.8% (95% CI 4.3–41.1) and 3.3% (91%CI 1.9–5.4), respectively (Fig. 3). In terms of cell of origin by Hans classifier, the risk of non-germinal center B-cell (GCB) type was relatively high (sHR 2.89, 95% CI 0.84–9.97, p = 0.094). Two-year cumulative incidence was 1.6% (95% CI 0.3–5.3) in patients with GCB type and 4.8% (95% CI 2.4–8.3) in those with non-GCB type. Kidney, adrenal gland, and testis are well known to be high-risk organs for sCNSi but were not evaluated in the present study because none of these lesions were observed at the time of registration in any patient with sCNSi.
Prognosis after the development of sCNSi
As of the point of data cutoff, 7 of the 21 patients who developed sCNSi survived. Within the median follow-up duration of 3.8 years after the development of sCNSi in the surviving patients, median survival time was 1.3 years, and 2-year OS was 39.3% (95% CI 17.4–60.7). In the 67 patients with non-CNS progression/relapse, median survival time from progression/relapse was 1.5 years, 2-year OS was 40.4% (95% CI 27.3–53.2), and there was no significant difference between the sCNSi group and the non-CNS progression or relapse group (p = 0.70) (Fig. 4 a). Furthermore, median survival time was 5.7 months (95% CI 3.2–5.9) in patients who developed sCNSi within 6 months after registration (sCNSi-POD6) and was 18.5 months (95% CI 12.2–66.1) thereafter (p < 0.0001), which suggests that patients with early development of sCNSi were resistant to subsequent therapies (Fig. 4 b).
Discussion
The present analysis examined sCNSi in patients treated with rituximab combined with CHOP as a supplementary analysis of JCOG0601, in which most patients were of lower IPI risk. The overall cumulative incidence of sCNSi of 5.1% was comparable with those of previous reports [1,2,3,4,5]. Of 21 patients who developed sCNSi, 8 and 10 were categorized as low and intermediate risk by CNS-IPI, respectively, and lesions in the paranasal cavity and orbital cavity were identified as having significantly high risk. Age > 60 years was also identified as a high-risk factor. In general, OS after sCNSi has been reported to be poor [4, 9], but the 2-year OS of 39.3% after the development of sCNSi was similar to that of non-CNS progression or relapse in JCOG0601.
The JCOG0601 compared standard R-CHOP-21 (arm A) with RW-CHOP-21 (arm B). The study demonstrated that PFS as the primary endpoint did not differ between the two arms, and could not confirm the superiority of dose-dense administration of rituximab at the start of treatment [18]. It is noteworthy that the number of patients with sCNSi was lower in arm B than in arm A, which requires careful interpretation. Compared to patients in arm A, many more patients in arm B were lower IPI risk (88% vs 76%), fewer were high IPI risk (4% vs 7%), and fewer were high CNS-IPI risk (4% vs 8%). Although the lack of patients with sCNSi in the high risk category of CNS-IPI in arm B might have led to this difference, the dose-dense administration of rituximab might have led to the lower number of patients with sCNSi in arm B.
In general, sCNSi in DLBCL had developed by 2 years after diagnosis [2, 5, 20]. In the present analysis, the time from registration to sCNSi by CNS-IPI ranged from 0.5 to 1.3 years among the risk categories, and the time to sCNSi was shortest in patients with high risk by CNS-IPI. These durations are comparable with those in previous studies [2, 20]. Intriguingly, three of the five patients who developed sCNSi at more than 2 years after registration did not have extranodal involvement at the time of registration. A possible explanation for this finding might be that a longer duration would be required for tumor cells in lymph nodes to develop a CNS lesion. In contrast, patients with early development of sCNSi, and particularly those with sCNSi-POD6, might have had subclinical CNS involvement at the time of registration. Clinical outcomes of these patients were extremely poor, similar to those reported in a recent study [21]. Given that patients with early-onset sCNSi developed the disease during the initial series of treatment and were quite resistant to subsequent therapies, biological differences in the nature of lymphoma cells between patients with early development and those with late onset should be investigated in the future.
The present analysis identified lesions in the paranasal cavity and orbital cavity as high risk for sCNSi. According to previous studies, the paranasal cavity is well known to be a high-risk organ [22,23,24], and the fact that sCNSi can occur in lower-risk patients by CNS-IPI might be associated with the proximity of the paranasal cavity and CNS. The orbital cavity is also well known as a high-risk organ [25]; however, only 1 of 2 patients with such a lesion developed sCNSi, which is too few to enable interpretation. We were also unable to confirm the significance of lesions in the kidney, adrenal gland, and testis, which are known to be high-risk organs [14, 20], because no patient with sCNSi had a lesion in these organs at the time of registration. This might be related to the low frequency of lesions occurring in these organs and because the study initially targeted patients with lower IPI risk.
In the present analysis, outcomes of patients with sCNSi and non-CNS progression were comparable. In previously reported analyses, outcomes of patients with non-CNS progression were extremely poor for the strategy of conventional salvage therapies plus high-dose therapy with autologous stem cell transplantation, especially in patients who were refractory to the initial series of treatment or who had relapsed disease within 12 months [26, 27]. Outcomes in these patients have shown remarkable improvement, from approximately 20% to 50%, by applying chimeric antigen receptor (CAR) T-cell therapy [28,29,30,31]. In contrast, in patients with sCNSi, several prospective studies have indicated that the outcomes of patients who could receive high-dose therapy with autologous stem cell transplantation after high-dose methotrexate-based regimen were comparable to those of patients with non-CNS progression [32,33,34]. This suggests that active treatment is desirable for younger patients with sCNSi who respond to salvage therapies. In terms of elderly patients, CAR T-cell therapy is becoming widely applied to patients with non-CNS progression. However, the effectiveness of CAR T-cell therapy is limited in patients with sCNSi [35]. Further development of treatment for elderly patients is required.
Finally, we should discuss the limitations of this analysis. The present study is a supplementary analysis based on the JCOG0601, which ensured a uniform population and protocol treatment. However, sCNSi data were available only at the first progression or at relapse, and were not available after the second or later progression or relapse. Therefore, the overall cumulative incidence might have been underestimated. In addition, information regarding treatment after sCNSi was insufficient, despite the clear importance of treatment after sCNSi. Nonetheless, the present study could provide basic information regarding sCNSi.
Conclusions
In conclusion, although the risk of sCNSi in patients at lower risk by CNS-IPI who were treated with rituximab combined with CHOP was low, it is important to pay attention to the development of sCNSi in patients with paranasal cavity lesions, even in those at lower risk by CNS-IPI.
Data availability
Individual participant data that underlie the results reported in this article will not be shared because the follow-up of the patients is continued until Dec. 2022. After the publication using data as of Dec. 2022, individual participant data that underlie the results after deidentification will be shared if investigators whose proposed use of the data has been approved by the investigators from Lymphoma Study Group of JCOG identified for this purpose. Proposals should be directed to 8jmmd004@is.icc.u-tokai.ac.jp. The data will be available for achieving aims in the approved proposal.
References
Villa D, Connors JM, Shenkier TN et al (2010) Incidence and risk factors for central nervous system relapse in patients with diffuse large B-cell lymphoma: the impact of the addition of rituximab to CHOP chemotherapy. Ann Oncol 21:1046–1052. https://doi.org/10.1093/annonc/mdp432
Klanova M, Sehn LH, Bence-Bruckler I et al (2019) Integration of cell of origin into the clinical CNS International Prognostic Index improves CNS relapse prediction in DLBCL. Blood 133:919–926. https://doi.org/10.1182/blood-2018-07-862862
Gleeson M, Counsell N, Cunningham D et al (2017) Central nervous system relapse of diffuse large B-cell lymphoma in the rituximab era: results of the UK NCRI R-CHOP-14 versus 21 trial. Ann Oncol 28:2511–2516. https://doi.org/10.1093/annonc/mdx353
Tomita N, Yokoyama M, Yamamoto W et al (2012) Central nervous system event in patients with diffuse large B-cell lymphoma in the rituximab era. Cancer Sci 103:245–251. https://doi.org/10.1111/j.1349-7006.2011.02139.x
Boehme V, Schmitz N, Zeynalova S et al (2009) CNS events in elderly patients with aggressive lymphoma treated with modern chemotherapy (CHOP-14) with or without rituximab: an analysis of patients treated in the RICOVER-60 trial of the German High-Grade Non-Hodgkin Lymphoma Study Group (DSHNHL). Blood 113:3896–3902. https://doi.org/10.1182/blood-2008-10-182253
Villa D, Connors JM, Sehn LH et al (2011) Diffuse large B-cell lymphoma with involvement of the kidney: outcome and risk of central nervous system relapse. Haematologica 96:1002–1007. https://doi.org/10.3324/haematol.2011.041277
Savage KJ, Slack GW, Mottok A et al (2016) Impact of dual expression of MYC and BCL2 by immunohistochemistry on the risk of CNS relapse in DLBCL. Blood 127:2182–2188. https://doi.org/10.1182/blood-2015-10-676700
Savage KJ (2017) Secondary CNS relapse in diffuse large B-cell lymphoma: defining high-risk patients and optimization of prophylaxis strategies. Hematol Am Soc Hematol Educ Program 2017:578–586. https://doi.org/10.1182/asheducation-2017.1.578
El-Galaly TC, Cheah CY, Bendtsen MD et al (2018) Treatment strategies, outcomes and prognostic factors in 291 patients with secondary CNS involvement by diffuse large B-cell lymphoma. Eur J Cancer 93:57–68. https://doi.org/10.1016/j.ejca.2018.01.073
Eyre TA, Savage KJ, Cheah CY et al (2022) CNS prophylaxis for diffuse large B-cell lymphoma. Lancet Oncol 23:e416–e426. https://doi.org/10.1016/S1470-2045(22)00371-0
Wilson MR, Eyre TA, Kirkwood AA et al (2022) Timing of high-dose methotrexate CNS prophylaxis in DLBCL: a multicenter international analysis of 1384 patients. Blood 139:2499–2511. https://doi.org/10.1182/blood.2021014506
Cheah CY, Herbert KE, O’Rourke K et al (2014) A multicentre retrospective comparison of central nervous system prophylaxis strategies among patients with high-risk diffuse large B-cell lymphoma. Br J Cancer 111:1072–1079. https://doi.org/10.1038/bjc.2014.405
Ferreri AJ, Bruno-Ventre M, Donadoni G et al (2015) Risk-tailored CNS prophylaxis in a mono-institutional series of 200 patients with diffuse large B-cell lymphoma treated in the rituximab era. Br J Haematol 168:654–662. https://doi.org/10.1111/bjh.13194
Zucca E, Conconi A, Mughal TI et al (2003) Patterns of outcome and prognostic factors in primary large-cell lymphoma of the testis in a survey by the International Extranodal Lymphoma Study Group. J Clin Oncol 21:20–27. https://doi.org/10.1200/JCO.2003.11.141
Vitolo U, Chiappella A, Ferreri AJ et al (2011) First-line treatment for primary testicular diffuse large B-cell lymphoma with rituximab-CHOP, CNS prophylaxis, and contralateral testis irradiation: final results of an international phase II trial. J Clin Oncol 29:2766–2772. https://doi.org/10.1200/JCO.2010.31.4187
Shimada K, Murase T, Matsue K et al (2010) Central nervous system involvement in intravascular large B-cell lymphoma: a retrospective analysis of 109 patients. Cancer Sci 101:1480–1486. https://doi.org/10.1111/j.1349-7006.2010.01555.x
Shimada K, Yamaguchi M, Atsuta Y et al (2020) Rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone combined with high-dose methotrexate plus intrathecal chemotherapy for newly diagnosed intravascular large B-cell lymphoma (PRIMEUR-IVL): a multicentre, single-arm, phase 2 trial. Lancet Oncol 21:593–602. https://doi.org/10.1016/S1470-2045(20)30059-0
Ohmachi K, Kinoshita T, Tobinai K et al (2021) A randomized phase 2/3 study of R-CHOP vs CHOP combined with dose-dense rituximab for DLBCL: the JCOG0601 trial. Blood Adv 5:984–993. https://doi.org/10.1182/bloodadvances.2020002567
Shipp MA, Harrington DP (1993) A predictive model for aggressive non-Hodgkin’s lymphoma. The International Non-Hodgkin’s Lymphoma Prognostic Factors Project. N Engl J Med 329:987–994. https://doi.org/10.1056/NEJM199309303291402
Schmitz N, Zeynalova S, Nickelsen M et al (2016) CNS International Prognostic Index: A Risk Model for CNS Relapse in Patients With Diffuse Large B-Cell Lymphoma Treated With R-CHOP. J Clin Oncol 34:3150–3156. https://doi.org/10.1200/JCO.2015.65.6520
Treiber H, Nilius-Eliliwi V, Seifert N et al (2023) Treatment Strategies and Prognostic Factors in Secondary Central Nervous System Lymphoma: A Multicenter Study of 124 Patients. Hemasphere 7:e926. https://doi.org/10.1097/HS9.0000000000000926
Boehme V, Zeynalova S, Kloess M et al (2007) Incidence and risk factors of central nervous system recurrence in aggressive lymphoma–a survey of 1693 patients treated in protocols of the German High-Grade Non-Hodgkin’s Lymphoma Study Group (DSHNHL). Ann Oncol 18:149–157
Hollender A, Kvaloy S, Nome O et al (2002) Central nervous system involvement following diagnosis of non-Hodgkin’s lymphoma: a risk model. Ann Oncol 13:1099–1107
Mian M, Capello D, Ventre MB et al (2014) Early-stage diffuse large B cell lymphoma of the head and neck: clinico-biological characterization and 18 year follow-up of 488 patients (IELSG 23 study). Ann Hematol 93:221–231. https://doi.org/10.1007/s00277-013-1856-4
Laskin JJ, Savage KJ, Voss N et al (2005) Primary paranasal sinus lymphoma: natural history and improved outcome with central nervous system chemoprophylaxis. Leuk Lymphoma 46:1721–1727. https://doi.org/10.1080/17402520500182345
Gisselbrecht C, Glass B, Mounier N et al (2010) Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. J Clin Oncol 28:4184–4190. https://doi.org/10.1200/JCO.2010.28.1618
Crump M, Neelapu SS, Farooq U et al (2017) Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study. Blood 130:1800–1808. https://doi.org/10.1182/blood-2017-03-769620
Locke FL, Miklos DB, Jacobson CA et al (2022) Axicabtagene Ciloleucel as Second-Line Therapy for Large B-Cell Lymphoma. N Engl J Med 386:640–654. https://doi.org/10.1056/NEJMoa2116133
Westin JR, Oluwole OO, Kersten MJ et al (2023) Survival with Axicabtagene Ciloleucel in Large B-Cell Lymphoma. N Engl J Med 389:148–157. https://doi.org/10.1056/NEJMoa2301665
Kamdar M, Solomon SR, Arnason J et al (2022) Lisocabtagene maraleucel versus standard of care with salvage chemotherapy followed by autologous stem cell transplantation as second-line treatment in patients with relapsed or refractory large B-cell lymphoma (TRANSFORM): results from an interim analysis of an open-label, randomised, phase 3 trial. Lancet 399:2294–2308. https://doi.org/10.1016/S0140-6736(22)00662-6
Abramson JS, Solomon SR, Arnason J et al (2023) Lisocabtagene maraleucel as second-line therapy for large B-cell lymphoma: primary analysis of the phase 3 TRANSFORM study. Blood 141:1675–1684. https://doi.org/10.1182/blood.2022018730
Ferreri AJ, Donadoni G, Cabras MG et al (2015) High Doses of Antimetabolites Followed by High-Dose Sequential Chemoimmunotherapy and Autologous Stem-Cell Transplantation in Patients With Systemic B-Cell Lymphoma and Secondary CNS Involvement: Final Results of a Multicenter Phase II Trial. J Clin Oncol 33:3903–3910. https://doi.org/10.1200/JCO.2015.61.1236
Ferreri AJM, Doorduijn JK, Re A et al (2021) MATRix-RICE therapy and autologous haematopoietic stem-cell transplantation in diffuse large B-cell lymphoma with secondary CNS involvement (MARIETTA): an international, single-arm, phase 2 trial. Lancet Haematol 8:e110–e121. https://doi.org/10.1016/S2352-3026(20)30366-5
Korfel A, Elter T, Thiel E et al (2013) Phase II study of central nervous system (CNS)-directed chemotherapy including high-dose chemotherapy with autologous stem cell transplantation for CNS relapse of aggressive lymphomas. Haematologica 98:364–370. https://doi.org/10.3324/haematol.2012.077917
Ahmed G, Hamadani M, Shah NN (2021) CAR T-cell therapy for secondary CNS DLBCL. Blood Adv 5:5626–5630. https://doi.org/10.1182/bloodadvances.2021005292
Acknowledgements
The authors wish to express their gratitude to pathologists Dr. Yoshihiro Matsuno and Dr. Naoya Nakamura for their support in the Central Pathological Review, and to the members of the JCOG Data Center and JCOG Operations Office for their support in preparing the poster (Dr. Tomohiro Kadota, Dr. Tomoko Kataoka), in data management (Ms. Yuko Watanabe), and for management and oversight of the study (Dr. Haruhiko Fukuda). Participating institutions (48 institutions): Ohta Nishinouchi Hospital, Tokyo Metropolitan Cancer and Infectious Diseases Center Komagome Hospital, Cancer Institute Hospital of the Japanese Foundation for Cancer Research, Tokyo Medical University Hospital, Aichi Medical University, Japanese Red Cross Nagasaki Genbaku Hospital, Japanese Red Cross Nagoya Daini Hospital, Sasebo City General Hospital, Shiga General Hospital, Oita Prefectural Hospital, Tokai University School of Medicine, Toyota Kosei Hospital, National Hospital Organization Hokkaido Cancer Center, Nagasaki Medical Center, University of Occupational and Environmental Health, Jikei University, Daisan Hospital, Gunma University, Imamura General Hospital, University of Fukui Hospital, Kyorin University Faculty of Medicine, National Kyushu Cancer Center, Nagasaki University Hospital, Nagoya City University Hospital, Chiba Cancer Center, Saitama Cancer Center, NTT Medical Center Tokyo, Shimane University Faculty of Medicine, Kagoshima University Hospital, Sapporo Hokuyu Hospital, Tohoku University Hospital, National Hospital Organization Nagoya Medical Center, Nagoya University School of Medicine, Hyogo Cancer Center, Jikei University Hospital, Hamamatsu University School of Medicine, National Hospital Organization Shikoku Cancer Center, NHO Kumamoto Medical Center, Mie University School of Medicine, Kyoto Prefectural University of Medicine, Faculty of Medicine, Saga University, Kumamoto University Medical School, National Cancer Center Hospital, National Hospital Organization Kyushu Medical Center, Aichi Cancer Center Hospital, School of Medicine, Fukuoka University, Akita University Graduate School of Medicine, Ehime University Hospital, Yamagata University Hospital, National Cancer Center Hospital East, Saitama Medical Center, Saitama Medical University, Saitama Medical University International Medical Center, Kindai University Faculty of Medicine, Kanazawa Medical University
Funding
Open Access funding provided by Nagoya University. The study was supported in part by the National Cancer Center Research and Development Fund (23-A-16, 23-A-17, 26-A-4, 29-A-3, 2020-J-3, 2023-J-03), Grants-in-Aid for Cancer Research (20S-1, 20S-6, 23-A-16, 23-A-17) and Health and Labour Sciences Research Grant for Clinical Cancer Research (19–20 and 22–14) from the Ministry of Health, Labour and Welfare.
Author information
Authors and Affiliations
Contributions
KS, KO, RM, DM, TK, KA, KT, and HN designed the study and wrote the manuscript. KS, KO, SO, HI, HT, NT, T Shima, MK, TT, T Shimo, DM, TK, KA, KT, and HN treated patients and obtained data. KS, KO, RM, DM, and HN analyzed and interpreted data. RM performed statistical analysis. KO prepared and diagnosed pathological specimens for central pathological review. TK, KA, TH, KT, and HN obtained financial support and supervised the research. All authors have read and approved the final version of the report.
Corresponding author
Ethics declarations
Ethical approval
The study was done in accordance with the Declaration of Helsinki, the Ethical guideline for clinical research issued by the Ministry of Health, Labour and Welfare in Japan, and the Clinical Trials Act in Japan. The study protocol was approved by the Protocol Review Committee of Japan Clinical Oncology Group (JCOG) and the institutional review board of each participating center and the central review board of National Cancer Center (Tokyo, Japan), since enforcement of the Clinical Trials Act in Japan.
Informed consent
Written informed consent was obtained from all patients before enrollment of the study.
Competing interests
KS reported receiving grants and personal fees from Celgene, and Kyowa Kirin, grants from Otsuka, and personal fees from AstraZeneca, Eisai, Takeda, Janssen, Bristol Myers Squibb, Chugai, Nippon Shinyaku, Daiichi Sankyo, Meiji Seika, Ono, AbbVie, Novartis, Gilead, CSL Behling, Incyte, and Genmab outside the submitted work. KO reported receiving personal fees from Chugai, Kyowa Kirin, Meiji Seika, and Pfizer outside the submitted work. SO reported receiving personal fees from Novartis, Bristol Myers Squibb, Takeda, AstraZeneca, Janssen, and AbbVie outside the submitted work. NT reported receiving personal fees from Chugai and Kyowa Kirin outside the submitted work. TS reported receiving personal fees from Chugai, Novartis, Bristol Myers Squibb, Kyowa Kirin, and Taiho outside the submitted work. KM reported receiving grants and personal fees from Eisai, Nippon Shinyaku, Chugai, Asahi Kasei, and Sumitomo Dainippon, grants from Takeda, Otsuka, and Zenyaku, and personal fees from SymBio, Janssen, AstraZeneca, Bristol Myers Squibb, Meiji Seika, AbbVie, Novartis, and Incyte outside the submitted work. DM reported receiving grants and personal fees from AbbVie, Celgene, Kyowa Kirin, Chugai, Ono, Takeda, Janssen, Sanofi, Bristol Myers Squibb, Eisai, and MSD, grants from Amgen Astellas Biopharma, Novartis, Otsuka, Astellas, and Taiho, and personal fees from Nippon Shinyaku, Mundipharma, Zenyaku, SymBio, and AstraZeneca outside the submitted work. KA reported receiving grants from Chugai, Nippon Shinyaku, Takeda, Eisai, and Kyowa Kirin, and personal fees from Celgene, Otsuka, Novartis, and Astellas outside the submitted work. KT reported receiving grants from National Cancer Center and the Ministry of Health, Labour and Welfare during the conduct of the study, grants and personal fees from Meiji Seika, Daiichi Sankyo, and HUYABIO, grants from Kyowa Kirin, Bristol Myers Squibb, Bayer, and Regeneron, and personal fees from Ono, Solasia, Yakuruto, Eisai, and Takeda outside the submitted work. HN reported receiving grants and personal fees from AstraZeneca, Celgene, Mundipharma, Takeda, Chugai, Bristol Myers Squibb, Jansen, Kyowa Kirin, SymBio, Ono, and Eisai, grants from Zenyaku Kogyo, Bayer, AbbVie, MSD, and Nippon Shinyaku, and personal fees from Sanofi, Novartis, Chordia Therapeutics, Nihon Medi-Physics, and Meiji Seika outside the submitted work. All other authors have nothing to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shimada, K., Ohmachi, K., Machida, R. et al. Secondary central nervous system involvement in patients with diffuse large B-cell lymphoma treated with rituximab combined CHOP therapy – a supplementary analysis of JCOG0601. Ann Hematol 103, 2021–2031 (2024). https://doi.org/10.1007/s00277-024-05620-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-024-05620-3