Abstract
Epstein-Barr virus (EBV) reactivation can occur following allogenic hematopoietic stem cell transplantation (allo-HSCT). However, the clinical characteristics and outcomes of EBV-viral load are not well known. Thus, we retrospectively analyzed the clinical features and prognostic impact of the EBV viral load in 121 allo-HSCT recipients from our hospital. EBV DNA quantification was performed in whole blood after transplantation. Patients were grouped based on whether EBV DNA quantification reached > 1000 copies/mL during follow-up (N = 50) or not (N = 71). Patients with EBV > 1000 EBV copies/mL were relatively more common in the groups with graft versus host disease (GVHD) prophylaxis including ATG, haploidentical donor type, peripheral blood as a donor source, and acute GVHD II–IV. The 20-month OS and DFS were not significantly different between patients with < 1000 EBV copies/mL and patients with > 1000 EBV copies/mL (20-month OS, 56.0% vs. 60.6%; p = 0.503, 20-month DFS, 50.0% vs. 57.7%; p = 0.179). Immunosuppressant (ISS) dose reduction was achieved after the maximum increase in EBV in 41/50 (82%) patients. Additionally, 30/50 (60%) patients achieved a 50% dose reduction or no restarting of ISS within 3 months of the maximum EBV increase. Among cases wherein EBV DNA quantification reached > 1000 copies/mL, those that achieved rapid dose reduction of ISS tended to have longer overall survival (“not reached” vs 5.4 months, p < 0.001) and disease-free survival (88.4 months vs 5.3 months, p < 0.001) than those in patients who did not. Our data highlight the importance of rapid ISS reduction in post-transplant EBV reactivation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Epstein-Barr virus (EBV) is the causative agent of infectious mononucleosis. EBV remains latent in lymphocytes after the initial infection and often reactivates after allogeneic hematopoietic stem cell transplantation (allo-HSCT) [1]. Occasionally, EBV reactivation progresses to lymphoproliferative disorder (LPD), and the types of LPD range from benign polyclonal B cell proliferation to malignant B/T cell lymphoma [2] [3]. LPD that occurs after allo-HSCT is of donor origin [4, 5].
The incidence of post-transplant lymphoproliferative disorders (PTLD) after allo-HSCT is approximately 1.0% [2]. The time of onset peaks between 60 and 90 days after HSCT, with 73% of cases occurring between 1 month and less than 6 months after HSCT and 9% between 6 months and up to 1 year. No cases occurred less than 1 month after HSCT, and cases rarely occurred after 1 year [2].
Risk factors for EBV reactivation include pre-transplant risk factors such as T cell depletion, EBV serology donor/recipient mismatch, cord blood transplantation, HLA mismatch, and second HSCT. Post-transplant risk factors include acute or chronic graft versus host disease (GVHD) requiring intensive immunosuppressant (ISS), high viral load, and mesenchymal stem cell therapy [6].
The European Conference on Infectious Diseases of Leukemia recommends weekly screening for EBV DNA in allo-HSCT recipients for at least 3 months after allo-HSCT [6].
A definitive diagnosis of LPD can be made by biopsy; however, this is difficult depending on the patient’s general condition after allo-HSCT. Monitoring EBV DNA in the peripheral blood is helpful in predicting the development of LPD [7]. The threshold value of EBV load can range from 1000 to over 40,000 copies/mL, depending on the sample, such as plasma, whole blood, and serum [8,9,10,11,12,13].
Due to the lack of direct treatment of EBV at the time of reactivation, prevention, ISS dose reduction, and preemptive treatment at the time of reactivation are important. The benefit of preemptive rituximab therapy is particularly notable in cases where EBV-infected cells are B cells. In contrast, rituximab can worsen the immunocompromised status after transplantation, thereby increasing the risk of infection and other complications. Reducing ISS dose is an important treatment for EBV reactivation; however, there are few established measures for ISS dose reduction, and the prognostic value of reduced ISS use has not been reported.
This study retrospectively analyzed the clinical features and prognostic impact of EBV viral load in allo-HSCT recipients.
Methods
Patient selection
This was a single-center, retrospective, observational study. A total of 121 consecutive patients who underwent allo-HSCT between December 2011 and February 2022 and EBV DNA quantification post-transplantation were included. Data on patients’ clinical findings were obtained from electronic medical records. The dataset was locked on February 28, 2022. Only cases in which the EBV viral load was measured over time after transplantation were included. This study was conducted in accordance with the Declaration of Helsinki 1964 and its later amendments or comparable ethical standards. This was approved by the Ethical Review Committee of the Japanese Red Cross Narita Hospital. This study was a retrospective observational study and obtaining written consent was not mandatory. All participants did not express any refusal to the study documents published on the Web using the opt-out method. The opt-out method was approved by our institutional review board.
EBV viral load measurement and identification of EBV-infected cells
Antibody titers of EBV prior to transplantation were checked to confirm previous infections in all patients. EBV DNA was quantified in whole blood. The basic measurement frequency is once every 2 weeks or once a week until 100 days post-transplant, and once a month after 100 days. On the other hand, in cases with frequent visits or those receiving large amounts of immunosuppressants continuously due to GVHD, EBV was sometimes measured weekly, even after 100 days post-transplantation.
In some cases, EBV-infected cell identification analysis of peripheral blood was performed using multicolor flow cytometry (FCM) to test whether the infected cells were of B or T/NK cell origin. Identification of infected cells by FCM was performed at the discretion of the attending physician. Specifically, FCM is used to test for the loss of CD20 expression for the identification of EBV-infected cells. This was performed in some cases that did not respond well to rituximab.
Background diseases and donor sources
Diseases that required and actually performed transplantation were included in the study. Malignant diseases included leukemia, myelodysplastic syndrome, myeloproliferative neoplasms, and malignant lymphoma, while benign diseases included aplastic anemia.
Patients who underwent allo-HSCT for chronic active EBV infection and EBV-HLH, wherein EBV initially infected T/NK cells, were excluded. Donor sources included bone marrow, peripheral blood stem cells, and cord blood (CB). There were no 8/8 HLA match in CB and all CB were counted as mismatched unrelated donor (MMUD) for donor type.
Study definitions and endpoints
We grouped patients on whether EBV DNA levels reached > 1000 copies/mL in whole blood during follow-up (N = 50) or not (N = 71). Previous reports have used a cutoff of 1000 to over 40,000 copies/mL, which is an extensive range, and we used 1000 copies/mL as the cutoff to examine trends in a larger number of patients. We analyzed the relationship between the rate of ISS reduction and prognosis for the group that had > 1000 copies/mL. We also examined the ISS dose that could be reduced after the EBV viral load reached its maximum level. In particular, we examined whether existing ISS could be reduced to 50% of the dose within 3 months, considering the period when post-transplant EBV-LPD (PT-EBV-LPD) is most likely to occur late in the course of post-allo-HSCT. Even if the ISS dose could be reduced by 50% within 3 months, if the dose was subsequently increased after dose reduction, it was considered a case of immunosuppressive dose reduction failure. Although calcineurin inhibitors were the main immunosuppressive drugs, we also assessed whether the dosage of steroids and MMF could be reduced by 50% in 3 months. If the dosage was not reduced by 50%, it was considered as an ISS dose reduction failure. The decision to reduce the ISS dose was at the physician’s discretion and undertaken considering the EBV-viral load status, GVHD status, infection, and risk of disease recurrence. As a rule, ISS dose reductions were actively implemented when GVHD findings improved or disappeared, when severe viral infections occurred, or when signs of disease recurrence appeared. The use of rituximab was considered when the EBV viral load was particularly high, such as > 100,000 copies/mL, but it has also been used in cases of aggressive LPD like malignant lymphoma.
Disease-free survival (DFS) was calculated as the time interval from the date of transplantation to the date of first progression, recurrence, or death without progression. Overall survival (OS) was calculated as the time interval from the date of transplantation to the date of death from any cause.
Statistical analysis
Baseline clinical characteristics were compared between the groups using the Mann–Whitney U test for continuous variables and Fisher’s exact test for categorical variables. Survival was analyzed using the Kaplan–Meier method and compared using the log-rank test to evaluate OS and DFS. Statistical significance was set at p < 0.05. Aplastic anemia was excluded from the prognosis stratified by complete remission (CR)/not CR. Since there are many cases of early death after transplantation, OS/DFS analysis by the landmark method at 3 months/6 months was also performed in some analysis. The prognostic impact was evaluated using univariate Cox proportional hazard analyses. The Scatter Plot was used for assessing the relationship between the two continuous variables. All statistical analyses were conducted using the EZR software (Saitama Medical Center, Jichi Medical University) [14].
Results
Clinical characteristics of patients based on maximum EBV-DNA after allo-HSCT
The clinical characteristics of patients based on the presence of a high EBV viral load (> 1000 EBV copies/mL) are summarized in Table 1. The median age of all patients was 53.0 years, and 64.5% were male. EBV-DNA in 50 patients (41.3%) was > 1000 EBV copies/mL, while 71 (58.6%) did not. The underlying diseases were acute myeloid leukemia/myelodysplastic syndrome (68.6%), chronic myeloid leukemia (2.5%), myeloproliferative neoplasms (3.3%), acute lymphoblastic leukemia (16.5%), non-Hodgkin lymphoma/Hodgkin lymphoma (6.6%), and aplastic anemia (2.5%). Complete remission (CR) was achieved in 51.2% of the patients at the time of transplantation. Among patients with > 1000 EBV copies/mL, 48% achieved CR at the time of transplantation, compared to 53.5% in patients with < 1000 EBV copies/mL. There were no differences in conditioning, Eastern Cooperative Oncology Group (ECOG)- performance status (PS), chronic GVHD, or cytomegalovirus activation after transplantation between the patients with > 1000 EBV copies/mL and patients with < 1000 EBV copies/mL. In contrast, patients with > 1000 EBV copies/mL had a higher frequency of anti-thymocyte globulin (ATG) haplo-transplantation, acute GVHD (grade 2 or higher), and peripheral blood stem cells as the graft source.
Clinical characteristics of patients with > 1000 EBV copies/mL
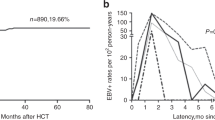
We then performed an additional analysis of the 50 patients with a high EBV viral load (> 1000 copies/mL) after transplantation (Table 2). Sixty percent of the patients reached the maximum EBV DNA level before post-transplant day 100, while 38% reached the maximum level between days 100 and 1000, and 2% reached maximum level after day 1000. The viral load was 3log (> 1000 copies/mL) in 52%, 4log (> 10,000 copies/mL) in 32%, 5log (> 100,000 copies/mL) in 8%, and 6log (> 1,000,000 copies/mL) in 8% of the patients. PT-EBV-LPD was diagnosed using lymph node biopsy or based on new multiple lymphadenopathies on CT in 10% of cases. The median viral load in patients with LPD was 1,500,000 EBV copies/mL (range 76,000–4,900,000 copies/mL). Tacrolimus and cyclosporine were used in 28% and 56% of the cases, respectively, when EBV reached the maximum viral load. Following maximum EBV increase, rituximab was used in six cases (12%), and 30 cases (60%) showed ISS dose reduction to 50% within 3 months. The percentage of patients with lymphocytes at least 1000/µL at maximum detection was 22/50 (44.0%). No correlation was observed between lymphocyte counts and maximum EBV-DNA levels, with a Spearman’s rank correlation coefficient − 0.066 (Online Resource.1).
Survival of allo-HSCT patients with EBV and ISS reduction
The median follow-up period was 16.2 (1.17–132.17/SD 34.6) months. EBV-PTLD began to appear as early as 3–6 months after transplantation in adults [15], and the previous report showed 3-year OS 47.3% for EBV-LPD after transplantation [16]; thus, we set the OS time point at approximately 20 months, which is close to the median observation period.
The median and 20-month OS rate of all patients were “not reached” and 58.7% (95% confidence interval [CI], 49.3–67.0%), respectively (Fig. 1A). The median and 20-month DFS rate of all patients were 74.8 months and 54.6% (95% CI, 45.1–63.0%), respectively (Fig. 1B). Patients who achieved CR before allo-HSCT showed significantly longer OS and DFS than those in patients who did not (20-month OS, 73.5% vs. 42.0% [95% CI, 60.4–82.9 vs. 28.9–54.6]; p = 0.0003; Fig. 2A; 20-month DFS, 68.3% vs. 38.5% [95% CI, 54.8–78.5 vs. 25.8–51.1]; p = 0.0002; Fig. 2B).
Patients with good PS (PS < 1) before allo-HSCT showed significantly longer OS and DFS than patients with poor PS (PS ≧ 1) before allo-HSCT (20-month OS, 69.0% vs. 45.1% [95% CI, 56.5–78.6 vs. 31.2–58.0]; p = 0.0189; Fig. 2C; and 20-month DFS, 65.8% vs. 39.2% [95% CI, 53.1–75.9 vs. 26.0–52.2]; p = 0.0021; Fig. 2D).
The 20-month OS (Fig. 3A) and DFS (Fig. 3B) were not significantly different between patients with < 1000 EBV copies/mL and patients with > 1000 EBV copies/mL (20-month OS, 56.0% vs. 60.6% [95% CI, 41.2–68.4% vs. 48.0–71.1%]; p = 0.503; 20-month DFS, 50.0% vs. 57.7% [95% CI, 35.6–62.8% vs. 45.0–68.4%]; p = 0.179).
Because patients could have died or showed progression/relapse before reaching their highest EBV viral load, a landmark OS analysis was performed at 3/6 months in the EBV viral load analysis. Patients with > 1000 EBV copies/mL were not significantly different in OS or DFS compared to patients with < 1000 EBV copies/mL (3-month landmark/20-month OS, 63.6% vs. 70.4%, p = 0.291, 3-month landmark/20-month DFS, 61.0% vs. 71.8%, p = 0.051, and 6-month landmark/20-month OS, 80.0% vs. 75.5%, p = 0.849, 6-month landmark/20-month DFS, 78.1% vs. 78.9%, p = 0.273) (Online Resource.2).
Further focusing on patients with > 1000 EBV copies/mL, those who achieved 50% ISS dose reduction, including not restarting ISS, within 3 months after HSCT did not have a significant difference in the frequency of chronic GVHD (10.0% vs. 15.0%; p = 0.672) or rituximab use (56.7% vs. 45.0%; p = 0.565) than those who did not achieve 50% dose reduction. However, these patients showed significantly longer OS and DFS than those who did not achieve 50% dose reduction (20-month OS, 80.0% vs. 20.0% [95% CI, 60.8–90.5 vs. 6.2–39.3]; p < 0.0001; Fig. 4A; and 20-month DFS, 70.0% vs. 20.0% [95% CI, 50.3–83.1 vs. 6.2–39.3]; p < 0.0001; Fig. 4B).
A OS and B DFS of patients with > 1000 EBV copies/mL based on the achievement of 50% dose reduction or no restarting of ISS within 3 months after the maximum EBV increase. C OS and D DFS based on the presence of a maximum EBV increase < 100 days after HSCT in patients with > 1000 EBV copies/mL. DFS, disease-free survival; EBV, Epstein-Barr virus; HSCT, hematopoietic stem cell transplantation; ISS, immunosuppressants; OS, overall survival
In considering dose reduction of ISS, an additional landmark analysis was performed because there were cases of death/progression before ISS dose reduction. Additional analysis focused only on patients who developed EBV reactivation, particularly within 12 months of transplantation. Those who achieved 50% ISS dose reduction showed significantly longer OS and DFS than those who did not achieve 50% dose reduction (3-month landmark/median OS, NR vs. 6.9 months, p = 0.001, 3-month landmark/median DFS, 88.4 months vs. 7.6 months, p = 0.007, and 6-month landmark/median OS, NR vs. 12.6 months, p = 0.025, 6-month landmark median DFS, 88.4 months vs. 12.1 months, p = 0.018) (Online Resource. 3).
Rituximab was used in 10% (3/30) of patients in the group that achieved rapid ISS reduction and in 15% (3/20) in the group that did not. There were two deaths due to EBV-LPD, and a rapid ISS dose reduction could not be achieved; however, rituximab was administered. Patients who achieved a 50% ISS dose reduction, including not restarting ISS, within 3 months, were able to achieve a 1 or 2log reduction in EBV DNA levels than those who did not achieve 50% dose reduction (86.7% vs. 55.0%; p = 0.021).
Patients who reached the maximum EBV-DNA level over 100 days post-transplantation had better OS and DFS than those who reached the maximum EBV DNA level within 100 days post-transplantation (20-month OS, 75.0% vs. 43.3% [95% CI, 50.0–88.7 vs. 25.6–59.9]; p = 0.015; Fig. 4C; and 20-month DFS, 73.5% vs. 43.3% [95% CI, 47.5–88.1 vs. 25.6–59.9]; p = 0.013; Fig. 4D).
Univariate Cox regression analysis of OS and DFS
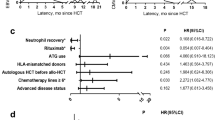
Univariate analysis was performed to identify factors independently associated with survival in patients with > 1000 EBV copies/mL after transplantation (Table 3). In the univariate analysis, achieving 50% dose reduction or not restarting ISS within 3 months after the maximum EBV increase, PS, and disease status were significantly associated with OS. ATG use, achieving 50% dose reduction or not restarting ISS within 3 months after the maximum EBV increase, and PS and disease status were significantly associated with DFS.
Characteristics of cases with high EBV viral load despite the end of ISS agents
In five cases, the amount of EBV DNA in the peripheral blood continued to increase even after ISS ended (Table 4). The age at transplantation ranged from 47 to 66 years, and the background diseases included acute lymphoblastic leukemia (one case), myeloproliferative neoplasms (one case), non-Hodgkin lymphoma (one case), and myelodysplastic syndrome (two cases). Conditioning included myeloablative conditioning in two cases and reduced intensity conditioning in three cases. GVHD prophylaxis included ATG in only one case, and no post-transplant cyclophosphamide haplo-transplantation was done. None of the patients had post-transplant relapse or progression to PT-EBV-LPD. All patients were followed up without rituximab. In all cases, EBV did not disappear, and a high viral load (> 10,000 copies/mL) was maintained.
Discussion
Allogeneic PT-EBV-LPD treatment includes rituximab treatment, reduction of ISS, donor lymphocyte infusion, and chemotherapy [6, 7, 15]. The benefits of rituximab are especially marked [16]. Since it is possible that LPD of T/NK cell types may be present, identification of EBV-infected cells using flow cytometry should also be undertaken [17].
The use of rituximab is an important component in the treatment of PT-EBV-LPD. Depending on the malignancy grade or CD20 expression in PTLD, there have been cases in which rituximab alone was not curative, including B cell LPD [18].
In our study, rituximab was used in some patients with high EBV viral loads, and CD20 expression decreased rapidly during rituximab treatment. The mechanism for this is known to involve the downregulation of surface CD20 [18]. In such cases, a combination of other chemotherapies, such as the CHOP regimen, may be effective. The efficacy of rituximab is more limited for EBV-LPDs localized in the central nervous system because of the blood–brain barrier more than other regions. Hence, treatment strategies other than rituximab should be considered [19]. A special approach involving intrathecal rituximab has been reported for the central nervous system PT-EBV-LPD. However, this approach has not been widely established [20].
A few established indicators for the specific rate of ISS dose reduction should be used in post-transplant EBV increase. In fact, there is only a small amount of literature examining specific methods of ISS reduction after EBV reactivation. Cesaro et al. reported a sustained decrease of at least 20% of the daily dose of ISS drugs for PT-EBV-LPD, except for low-dose corticosteroid therapy [21]. Hematopoiesis is sometimes unstable after transplantation, and chemotherapy for EBV is more difficult due to the decrease in hematopoiesis. Therefore, we believe that our strategy of ISS dose reduction by 50% within 3 months after EBV increase may be beneficial. Prevention and treatment of GVHD are key to the success of this strategy. Nevertheless, rituximab is also effective in the polyclonal phase, and a combination of rituximab therapy and the rapid reduction of ISS should be combined when EBV viral load is elevated.
In our study, the EBV viral load was not a prognostic factor. However, it has been suggested that rapidly reducing ISS dose may prevent PT-EBV-LPD. A reduction in EBV DNA levels was also predominant in patients who achieved rapid ISS dose reductions. Moreover, it is expected that chronic GVHD would not be as frequent in patients whose ISS were reduced to 50% within 3 months after increasing EBV; however, the frequency of chronic GVHD was not significantly different between the groups that had rapidly reduced ISS dose and those that did not. In cases with GVHD, it may be important to reduce ISS use according to the grade of GVHD; however, in cases without GVHD, a more aggressive and rapid ISS reduction may be helpful in controlling the current disease and preventing EBV reactivation. In our study, the prognosis may be worse in cases of an early increase in EBV after transplantation.
In our analysis, there were several cases of persistently high EBV viral load after allo-HSCT, despite the absence of ISS. The development of EBV-positive Hodgkin lymphoma has been previously reported in cases wherein CD4-positive lymphocytes in the peripheral blood recovered after HIV treatment [22]. These are cases of EBV-positive lymphoma in patients whose immune competence has recovered, and a different mechanism must be assumed for immunocompromised methotrexate-associated lymphproliferative disorder or other iatrogenic immunodeficiency-associated LPD. The recovery of CD4-positive T cells is expected to result in the increased production of various cytokines, wherein some of these T cell cytokines may act on the proliferation of the B cell lineage [23]. In our cases with some CD4 monitoring, there were some cases wherein recovery of CD4-positive lymphocytes was observed. Recovery of CD4-positive lymphocytes may be a factor in the cases with persistently high EBV viral load observed after completion of ISS. Surprisingly, these cases were uneventful even in patients who did not receive rituximab or other therapeutic chemotherapy.
The cohort in our study was a sample of patients for whom EBV could be measured over time after allo-HSCT—long-term survivors. This allowed for a relatively long observation period, and OS tended to be slightly better than prognosis of the typical allo-HSCT patient. However, the survival of this cohort has significant differences based on the presence of CR and good or bad PS and is not considered a cohort that deviates from the typical allo-HSCT patient.
CB and ATG-Haplo transplants are both risk factors for EBV reactivation. ATG-Haplo transplantation has been reported as a risk factor for increased EBV [24].
The relatively low proportion of patients who underwent CBT in the patients with > 1000 EBV copies/mL group may be attributed to the disproportionately high frequency of ATG-Haplo transplants in this group.
The limitations of this study include its small sample size and retrospective nature. The treatment was heterogeneous because of the long study period and the presence of various diseases. EBV DNA quantification in peripheral blood is not always helpful due to exceptions wherein PT-EBV-LPD develops without obvious EBV viremia and with low levels of EBV DNA in the blood [25]. Despite these limitations, to the best of our knowledge, this is the first study to describe a reduction in EBV reactivation and improved prognosis by rapid dose reduction of ISS after allo-HSCT.
In conclusion, we identified that after detecting a high EBV viral load, OS and DFS were significantly lower in the group of patients who could not reduce ISS use by 50% or less within 3 months than that in the group of patients who could. Although there have been several cases of long-term persistence of a high EBV viral load despite the end of ISS therapy, these cases were uneventful. To confirm our results, an independent study with larger and more detailed investigations with a prospective assessment is required.
References
Dunmire SK, Verghese PS, Balfour HH Jr (2018) Primary Epstein-Barr virus infection. J Clin Virol 102:84–92. https://doi.org/10.1016/j.jcv.2018.03.001
Curtis RE, Travis LB, Rowlings PA, Socié G, Kingma DW, Banks PM, Jaffe ES, Sale GE, Horowitz MM, Witherspoon RP, Shriner DA, Weisdorf DJ, Kolb HJ, Sullivan KM, Sobocinski KA, Gale RP, Hoover RN, Fraumeni JF Jr, Deeg HJ (1999) Risk of lymphoproliferative disorders after bone marrow transplantation: a multi-institutional study. Blood 94(7):2208–2216
Petrara MR, Giunco S, Serraino D, Dolcetti R, De Rossi A (2015) Post-transplant lymphoproliferative disorders: from epidemiology to pathogenesis-driven treatment. Cancer Lett 369(1):37–44. https://doi.org/10.1016/j.canlet.2015.08.007
Shapiro RS, McClain K, Frizzera G, Gajl-Peczalska KJ, Kersey JH, Blazar BR, Arthur DC, Patton DF, Greenberg JS, Burke B et al (1988) Epstein-Barr virus associated B cell lymphoproliferative disorders following bone marrow transplantation. Blood 71(5):1234–1243
Zutter MM, Martin PJ, Sale GE, Shulman HM, Fisher L, Thomas ED, Durnam DM (1988) Epstein-Barr virus lymphoproliferation after bone marrow transplantation. Blood 72(2):520–529
Styczynski J, van der Velden W, Fox CP, Engelhard D, de la Camara R, Cordonnier C, Ljungman P (2016) Management of Epstein-Barr virus infections and post-transplant lymphoproliferative disorders in patients after allogeneic hematopoietic stem cell transplantation: Sixth European Conference on Infections in Leukemia (ECIL-6) guidelines. Haematologica 101(7):803–811. https://doi.org/10.3324/haematol.2016.144428
Lindsay J, Yong MK, Greenwood M, Kong DCM, Chen SCA, Rawlinson W, Slavin M (2020) Epstein-Barr virus related post-transplant lymphoproliferative disorder prevention strategies in allogeneic hematopoietic stem cell transplantation. Rev Med Virol 30(4):e2108. https://doi.org/10.1002/rmv.2108
Wagner HJ, Cheng YC, Huls MH, Gee AP, Kuehnle I, Krance RA, Brenner MK, Rooney CM, Heslop HE (2004) Prompt versus preemptive intervention for EBV lymphoproliferative disease. Blood 103(10):3979–3981. https://doi.org/10.1182/blood-2003-12-4287
Deeg HJ, Storer BE, Boeckh M, Martin PJ, McCune JS, Myerson D, Heimfeld S, Flowers ME, Anasetti C, Doney KC, Hansen JA, Kiem HP, Nash RA, O’Donnell PV, Radich JP, Sandmaier BM, Scott BL, Sorror ML, Warren EH, Witherspoon RP, Woolfrey A, Appelbaum FR, Storb R (2006) Reduced incidence of acute and chronic graft-versus-host disease with the addition of thymoglobulin to a targeted busulfan/cyclophosphamide regimen. Biol Blood Marrow Transplant 12(5):573–584. https://doi.org/10.1016/j.bbmt.2005.12.036
Carpenter B, Haque T, Dimopoulou M, Atkinson C, Roughton M, Grace S, Denovan S, Fielding A, Kottaridis PD, Griffiths P, Mackinnon S, Emery V, Chakraverty R (2010) Incidence and dynamics of Epstein-Barr virus reactivation after alemtuzumab-based conditioning for allogeneic hematopoietic stem-cell transplantation. Transplantation 90(5):564–570. https://doi.org/10.1097/TP.0b013e3181e7a3bf
Worth A, Conyers R, Cohen J, Jagani M, Chiesa R, Rao K, Goulden N, Veys P, Amrolia PJ (2011) Pre-emptive rituximab based on viraemia and T cell reconstitution: a highly effective strategy for the prevention of Epstein-Barr virus-associated lymphoproliferative disease following stem cell transplantation. Br J Haematol 155(3):377–385. https://doi.org/10.1111/j.1365-2141.2011.08855.x
Wang Y, Fu HX, Liu DH, Xu LP, Zhang XH, Chang YJ, Chen YH, Wang FR, Sun YQ, Tang FF, Liu KY, Huang XJ (2014) Influence of two different doses of antithymocyte globulin in patients with standard-risk disease following haploidentical transplantation: a randomized trial. Bone Marrow Transplant 49(3):426–433. https://doi.org/10.1038/bmt.2013.191
Jain T, Kosiorek HE, Grys TE, Kung ST, Shah VS, Betcher JA, Slack JL, Leis JF, Khera N, Noel P, Palmer JM, Sproat LZ (2019) Single dose versus multiple doses of rituximab for preemptive therapy of Epstein-Barr virus reactivation after hematopoietic cell transplantation. Leuk Lymphoma 60(1):110–117. https://doi.org/10.1080/10428194.2018.1459603
Kanda Y (2013) Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant 48(3):452–458. https://doi.org/10.1038/bmt.2012.244
Compagno F, Basso S, Panigari A, Bagnarino J, Stoppini L, Maiello A, Mina T, Zelini P, Perotti C, Baldanti F, Zecca M, Comoli P (2020) Management of PTLD after hematopoietic stem cell transplantation: immunological perspectives. Front Immunol 11:567020. https://doi.org/10.3389/fimmu.2020.567020
Styczynski J, Gil L, Tridello G, Ljungman P, Donnelly JP, van der Velden W, Omar H, Martino R, Halkes C, Faraci M, Theunissen K, Kalwak K, Hubacek P, Sica S, Nozzoli C, Fagioli F, Matthes S, Diaz MA, Migliavacca M, Balduzzi A, Tomaszewska A, Camara Rde L, van Biezen A, Hoek J, Iacobelli S, Einsele H, Cesaro S (2013) Response to rituximab-based therapy and risk factor analysis in Epstein Barr virus-related lymphoproliferative disorder after hematopoietic stem cell transplant in children and adults: a study from the Infectious Diseases Working Party of the European Group for Blood and Marrow Transplantation. Clin Infect Dis 57(6):794–802. https://doi.org/10.1093/cid/cit391
Zhang P, Zeng C, Cheng J, Zhou J, Gu J, Mao X, Zhang W, Cao Y, Luo H, Xu B, Li Q, Xiao M, Zhou J (2019) Determination of Epstein-Barr virus-infected lymphocyte cell types in peripheral blood mononuclear cells as a valuable diagnostic tool in hematological diseases. Open Forum Infect Dis 6(5):ofz171. https://doi.org/10.1093/ofid/ofz171
Muramatsu H, Takahashi Y, Shimoyama Y, Doisaki S, Nishio N, Ito Y, Hama A, Shimada A, Yagasaki H, Ito M, Kojima S (2011) CD20-negative Epstein-Barr virus-associated post-transplant lymphoproliferative disease refractory to rituximab in a patient with severe aplastic anemia. Int J Hematol 93(6):779–781. https://doi.org/10.1007/s12185-011-0870-3
Batchelor TT, Grossman SA, Mikkelsen T, Ye X, Desideri S, Lesser GJ (2011) Rituximab monotherapy for patients with recurrent primary CNS lymphoma. Neurology 76(10):929–930. https://doi.org/10.1212/WNL.0b013e31820f2d94
Bonney DK, Htwe EE, Turner A, Kelsey A, Shabani A, Hughes S, Hughes I, Wynn RF (2012) Sustained response to intrathecal rituximab in EBV associated post-transplant lymphoproliferative disease confined to the central nervous system following haematopoietic stem cell transplant. Pediatr Blood Cancer 58(3):459–461. https://doi.org/10.1002/pbc.23134
Cesaro S, Pegoraro A, Tridello G, Calore E, Pillon M, Varotto S, Abate D, Barzon L, Mengoli C, Carli M, Messina C (2010) A prospective study on modulation of immunosuppression for Epstein-Barr virus reactivation in pediatric patients who underwent unrelated hematopoietic stem-cell transplantation. Transplantation 89(12):1533–1540. https://doi.org/10.1097/TP.0b013e3181dd6c0a
Lanoy E, Rosenberg PS, Fily F, Lascaux AS, Martinez V, Partisani M, Poizot-Martin I, Rouveix E, Engels EA, Costagliola D, Goedert JJ (2011) HIV-associated Hodgkin lymphoma during the first months on combination antiretroviral therapy. Blood 118(1):44–49. https://doi.org/10.1182/blood-2011-02-339275
Elfeky R, Lazareva A, Qasim W, Veys P (2019) Immune reconstitution following hematopoietic stem cell transplantation using different stem cell sources. Expert Rev Clin Immunol 15(7):735–751. https://doi.org/10.1080/1744666x.2019.1612746
van Esser JW, van der Holt B, Meijer E, Niesters HG, Trenschel R, Thijsen SF, van Loon AM, Frassoni F, Bacigalupo A, Schaefer UW, Osterhaus AD, Gratama JW, Löwenberg B, Verdonck LF, Cornelissen JJ (2001) Epstein-Barr virus (EBV) reactivation is a frequent event after allogeneic stem cell transplantation (SCT) and quantitatively predicts EBV-lymphoproliferative disease following T-cell–depleted SCT. Blood 98(4):972–978. https://doi.org/10.1182/blood.v98.4.972
Fox CP, Burns D, Parker AN, Peggs KS, Harvey CM, Natarajan S, Marks DI, Jackson B, Chakupurakal G, Dennis M, Lim Z, Cook G, Carpenter B, Pettitt AR, Mathew S, Connelly-Smith L, Yin JA, Viskaduraki M, Chakraverty R, Orchard K, Shaw BE, Byrne JL, Brookes C, Craddock CF, Chaganti S (2014) EBV-associated post-transplant lymphoproliferative disorder following in vivo T-cell-depleted allogeneic transplantation: clinical features, viral load correlates and prognostic factors in the rituximab era. Bone Marrow Transplant 49(2):280–286. https://doi.org/10.1038/bmt.2013.170
Acknowledgements
The authors would like to thank the patients who underwent allo-HSCT and the medical staff of the Department of Hematology and Oncology of the Japanese Red Cross Narita Hospital. We thank Ken-Ichi Imadome for the flow cytometry analysis of EBV-infected cells in some cases and Editage (https://www.editage.jp/) for English language editing. We also thank Toshiki Terao (Department of Hematology and Oncology, Okayama University Hospital) for advice from a statistical perspective.
Author information
Authors and Affiliations
Contributions
T.T. designed the study, collected the data, wrote the manuscript, and provided patient care. S.M., N.Y., S.K., C.K., S.K., K.T., K.M., S.S., K.K., H.A., Y.U., and N.A. provided patient care. All the authors have reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
All procedures were performed according to the ethical standards of the institutional and national research committee and the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This study was approved by the institutional review board.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tsushima, T., Masuda, SI., Yoda, N. et al. Clinical characteristics and outcomes of Epstein-Barr virus viral load after allogeneic hematopoietic stem cell transplantation. Ann Hematol 103, 935–946 (2024). https://doi.org/10.1007/s00277-023-05596-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-023-05596-6