Abstract
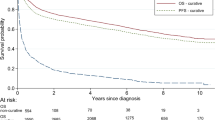
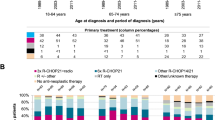
The COVID-19 pandemic posed a major challenge in cancer care worldwide which might have an impact on the management of diffuse large B-cell lymphoma (DLBCL). We conducted a retrospective study comparing characteristics, management, and outcomes of DLBCL patients diagnosed during the first year of the COVID-19 pandemic (1/3/2020–28/2/2021) to those diagnosed in the previous year (1/3/2019–28/2/2020) in two tertiary centers in Italy and Israel. 182 patients were diagnosed with DLBCL during the study period. More patients were diagnosed during the pandemic compared to the year before: 60 vs. 29 and 54 vs. 39 in Italy and in Israel, respectively. Trends towards older age and higher transformation rates were shown during the pandemic. The interval between the initiation of symptoms and diagnosis was longer during the pandemic. Five and four patients were diagnosed with COVID-19 during treatment in Italy and in Israel, respectively. there was no difference in dose density and intensity of treatment, before and during the pandemic. The median follow-up during and before the pandemic was 15.2 and 25.5 months, respectively. Progression-free survival (PFS) was slightly shorter during the pandemic compared to the year before (64.9% vs. 70.6%; p = 0.0499). In multivariate analysis, older age and transformed disease were independently related to PFS, while diagnosis of DLBCL during the pandemic was not. Despite the challenges caused by COVID-19 pandemic, the management of DLBCL patients remained unchanged including dose density and intensity. Nevertheless, a shorter PFS during the outbreak might be attributed to differences in patients’ characteristics.


Similar content being viewed by others
Data availability
The data supporting this study’s findings are available from the.
corresponding author, TB, upon reasonable request.
References
Teras LR, DeSantis CE, Cerhan JR, Morton LM, Jemal A, Flowers CR (2016) 2016 US lymphoid malignancy statistics by World Health Organization subtypes. CA Cancer J Clin 66(6):443–459
Lee S, Fujita K, Negoro E, Morishita T, Yamauchi H, Oiwa K et al (2019) The impact of diagnostic wait time on the survival of patients with diffuse large B-cell Lymphoma: effect modification by the International Prognostic Index. Br J Haematol 187(2):195–205
Gutierrez A, Bautista-Gili AM, Bento L, Herraez I, Garcia L, Martinez-serra J et al (2013) Impact of dose-density delays in diffuse large B-Cell Lymphoma (DLBCL) treated with R-CHOP21 or R-CHOP14. Blood 122(21):4374–4374
Shah V, Ko Ko T, Zuckerman M, Vidler J, Sharif S, Mehra V et al (2020) Poor outcome and prolonged persistence of SARS-CoV-2 RNA in COVID-19 patients with haematological malignancies; King’s College Hospital experience. Br J Haematol 190(5):e279–e282
Duléry R, Lamure S, Delord M, Di Blasi R, Chauchet A, Hueso T et al (2021) Prolonged in-hospital stay and higher mortality after Covid-19 among patients with non-hodgkin Lymphoma treated with B-cell depleting immunotherapy. Am J Hematol 96(8):934–944
Wells CR, Galvani AP (2022) Impact of the COVID-19 pandemic on cancer incidence and mortality. Lancet Public Health 7(6):e490–e491
Malagón T, Yong JHE, Tope P, Miller WH, Franco EL (2022) McGill Task Force on the impact of COVID-19 on Cancer Control and Care. Predicted long-term impact of COVID-19 pandemic-related care delays on cancer mortality in Canada. Int J Cancer 150(8):1244–1254
Jazieh AR, Akbulut H, Curigliano G, Rogado A, Alsharm AA, Razis ED et al (2020) Impact of the COVID-19 pandemic on Cancer Care: A Global Collaborative Study. JCO Glob Oncol 6:1428–1438
International Non-Hodgkin’s Lymphoma Prognostic Factors Project (1993) A predictive model for aggressive non-hodgkin’s Lymphoma. N Engl J Med 329(14):987–994
Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E et al (2014) Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-hodgkin Lymphoma: the Lugano classification. J Clin Oncol 32(27):3059–3068
Toh ZQ, Russell FM, Garland SM, Mulholland EK, Patton G, Licciardi PV (2021) Human papillomavirus vaccination after COVID-19. JNCI Cancer Spectr 5(2):pkab011
Luo Q, O’Connell DL, Yu XQ, Kahn C, Caruana M, Pesola F et al (2022) Cancer incidence and mortality in Australia from 2020 to 2044 and an exploratory analysis of the potential effect of treatment delays during the COVID-19 pandemic: a statistical modelling study. Lancet Public Health 7(6):e537–e548
Anandaradje A, Goenka L, Dubashi B, Kayal S, Halanaik D, Basu D et al (2021) ABCL-334: impact of COVID-19 pandemic on treatment duration in curable cancers: comparison of patients with DLBCL treated before and during: a retrospective record-based study. Clin Lymphoma Myeloma Leuk 21:S391
Ng DZ, Kang HX, Tan SH, Shih V, Zhuang Q, Chiang J et al (2020) 326P Management of diffuse large B cell Lymphomas in the COVID-19 era. Ann Oncol 31:S1368
Ferrara G, De Vincentiis L, Ambrosini-Spaltro A, Barbareschi M, Bertolini V, Contato E et al (2021) Cancer diagnostic delay in northern and central Italy during the 2020 lockdown due to the coronavirus Disease 2019 pandemic. Am J Clin Pathol 155(1):64–68
Lazzerini M, Barbi E, Apicella A, Marchetti F, Cardinale F, Trobia G (2020) Delayed access or provision of care in Italy resulting from fear of COVID-19. Lancet Child Adolesc Health 4(5):e10–e11
Lugli G, Ottaviani MM, Botta A, Ascione G, Bruschi A, Cagnazzo F et al (2022) The impact of the SARS-CoV-2 pandemic on Healthcare Provision in Italy to non-COVID patients: a systematic review. Mediterr J Hematol Infect Dis 14(1):e2022012
Islam N, Shkolnikov VM, Acosta RJ, Klimkin I, Kawachi I, Irizarry RA et al (2021) Excess deaths associated with covid-19 pandemic in 2020: age and sex disaggregated time series analysis in 29 high income countries. BMJ 373:n1137
Remuzzi A, Remuzzi G (2020) COVID-19 and Italy: what next? Lancet 395(10231):1225–1228
WHO Coronavirus (COVID-19) Dashboard | WHO Coronavirus (COVID-19) Dashboard With Vaccination Data [Internet]. [cited 2023 May 6]. Available from: https://covid19.who.int/
Chernichovsky D (2019) The healthcare system: an overview. Jerusalem Taub Center for Social Policy Studies in Israel
Ricciardi W, Tarricone R (2021) The evolution of the Italian National Health Service. Lancet 398(10317):2193–2206
Giorgi C (2023) A history of Italy’s health policy from the Republic to the new century. Mod Italy 28(1):1–17
Dagan N, Barda N, Kepten E, Miron O, Perchik S, Katz MA et al (2021) BNT162b2 mRNA Covid-19 vaccine in a Nationwide Mass Vaccination setting. N Engl J Med 384(15):1412–1423
Tarricone R, Listorti E, Tozzi V, Torbica A, Banks H, Ghislandi S et al (2021) Transformation of Cancer Care during and after the COVID pandemic, a point of no return. The experience of Italy. J Cancer Policy 29:100297
Funding
No funding was received for conduction this study.
Author information
Authors and Affiliations
Contributions
OG, OP and RG conceived the presented idea for the study and designed the research study. OG, GB, MG, TB, GI, GL, VS, AB LA and RG identified through databases DLBCL patients as potential candidates for the study and collected the data. OG, SS, LA and RG analysed the data. OG, AGG and RG drafted the manuscript. SS, OP, LA, PLZ contributed to the interpretation of results and along with SS, OP, PR, LA and PLZ critically revised the paper. All authors approved the submitted and final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Tamar Berger –Advisory committees: Janssen, Neopharm; (honoraria, advisory committee); honoraria: Janssen. Pia Raanan - Consultancy: Pfizer, Novartis, Bristol Myers Squibb; Advisory committee: Pfizer; Speaker bureau: Janssen; research support: Pfizer, Novartis. Pier Luigi Zinzani - Consultancy: BeiGene, Bristol-Myers Squibb, Takeda, EUSA Pharma, ADC Therapeutics, Merck Sharp & Dohme, Celgene, Celltrion, Kyowa Kirin, Roche, Verastem, Sandoz, Novartis, Incyte, TG Therapeutics Inc, Servier, Janssen-Cilag, Gilead, ImmuneDesign, Sanofi; Membership on an entity’s Board of Directors or advisory committees: BeiGene, Bristol-Myers Squibb, Takeda, EUSA Pharma, ADC Therapeutics, Merck Sharp & Dohme, Celgene, Celltrion, Kyowa Kirin, Roche, Verastem, Sandoz, Novartis, Incyte, TG Therapeutics Inc, Servier, Janssen-Cilag, Gilead, ImmuneDesign, Portola, Sanofi, AbbVie; Speakers Bureau: BeiGene, Bristol-Myers Squibb, Takeda, EUSA Pharma, Merck Sharp & Dohme, Celgene, Celltrion, Kyowa Kirin, Roche, Verastem, Sandoz, Novartis, Incyte, TG Therapeutics Inc, Servier, Janssen-Cilag, Gilead, ImmuneDesign, Portola, AbbVie; Honoraria: Bristol Myers Squibb, Takeda, EUSA Pharma, ADC Therapeutics, Merck Sharp & Dohme, Kyowa Kirin, Roche, Verastem, Incyte, TG Therapeutics Inc, Servier, Janssen-Cilag, AbbVie; Research funding: Portola. Ronit Gurion – Advisory committees: Roche, Gilead, Medison Ltd, Novartis, Abbvie; Honoraria: Roche, Gilead, Medison Ltd, Novartis, Abbvie and Takeda. All other authors have no relevant financial or non-financial interest to disclose.
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Approval was obtained from Rabin Medical Center (RMC) and Seragnoli Institutional Review Board (IRB) ethics committee.
Consent to participate
Informed consent was waived due to the retrospective design of the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Giladi, O., Bagnato, G., Gentilini, M. et al. Diffuse large B cell lymphoma characteristics and outcomes during the COVID-19 pandemic in two tertiary centers - an Israeli/ Italian study. Ann Hematol 103, 803–811 (2024). https://doi.org/10.1007/s00277-023-05543-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-023-05543-5