Abstract
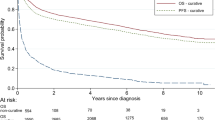
Relapsing diffuse large B cell lymphomas (rDLBCL) represent a heterogeneous disease. This heterogeneity should be recognized and reflected, because it can deform the interpretation of clinical trial results. DLBCL patients with the first relapse and without CNS involvement were identified in the Czech Lymphoma Study Group (CLSG) database. Interval-to-therapy (ITT) was defined as the time between the first manifestation of rDLBCL and the start of any treatment. The overall survival (OS) of different ITT cohorts (< 7 vs. 7–21 vs. > 21 days) was compared. In total, 587 rDLBCLs (51.8% males) progressed with a median of 12.8 months (range 1.6 to 152.3) since the initial diagnosis (2000–2017). At the time of relapse, the median age was 67 years (range 22–95). First-line therapy was administered in 99.3% of the patients; CHOP and anti-CD20 were given to 69.2% and 84.7% of the patients, respectively. The salvage immune/chemotherapy was administered in 88.1% of the patients (39.2% platinum-based regimen). The median ITT was 20 days (range 1–851), but 23.2% of patients initiated therapy within 7 days. The 5-year OS was 17.4% (range 10–24.5%) vs. 20.5% (range 13.5–27.4%) vs. 42.2% (range 35.5–48.8%) for ITT < 7 vs. 7–21 vs. > 21 days (p < 0.001). ITT was associated with B symptoms (p 0.004), ECOG (p < 0.001), stage (p 0.002), bulky disease (p 0.005), elevated LDH (p < 0.001), and IPI (p < 0.001). The ITT mirrors the real clinical behavior of rDLBCL. There are patients (ITT < 7 days) with aggressive disease and a poor outcome. Conversely, there are rDLBCLs with ITT ≥ 21 days who survive for a long time.





Similar content being viewed by others
References
Al-Hamadani M, Habermann TM, Cerhan JR et al (2015) Non-Hodgkin lymphoma subtype distribution, geodeomographic patterns, and survival in the US: a longitudinal analysis of the national cancer data base from 1998 to 2011. Am J Hematol 90:790–795
Habermann TM, Weller EA, Morisson VA et al (2006) Rituximab-CHOP versus CHOP alone or with maintenance rituximab in older patients with diffuse large B-cell lymphoma. J Clin Oncol 24:3121–3127
Pfreundschuh M, Trumpel L, Osterborg A et al (2006) CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large B-cell lymphoma: a randomised controlled trial by the MabThera International (MInT) group. Lancet Oncol 7:379–391
Sehn LH, Donaldson J, Chhanabhai M, Fitzgerald C, Gill K, Klasa R, MacPherson N, O'Reilly S, Spinelli JJ, Sutherland J, Wilson KS, Gascoyne RD, Connors JM (2005) Introduction of combined CHOP plus rituximab dramatically improved outcome of diffuse large B-cell lymphoma in British Columbia. J Clin Oncol 23:5027–5033
Howlander N, Mariotto AB, Besson C et al (2017) Cancer-specific mortality, cure fraction, and noncancer causes of death among diffuse large B-cell lymphoma patients in the immunochemotherapy era. Cancer 123:3326–3334
Coiffier B, Lepage E, Briére J et al (2002) CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. NEJM 346:235–242
Coiffier B, Thieblemont C, Den Van Neste E et al (2010) Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: a study by the Groupe d’Etudes des Lymphomes de l'Adulte. Blood 116:2040–2045
Farooq U, Maurer MJ, Thompson CA, Thanarajasingam G, Inwards DJ, Micallef I, Macon W, Syrbu S, Lin T, Lin Y, Ansell SM, Nowakowski GS, Habermann TM, Cerhan JR, Link BK (2017) Clinical heterogeneity of diffuse large B cell lymphoma following failure of front-line immunochemotherapy. Br J Haematol 179:50–60
Kahan BC, REhal S, Cro S (2015) Risk of selection bias in randomised trials. Trials 16:405. https://doi.org/10.1186/s13063-015-0920-x
Maurer MJ, Ghesquiéres H, Link BK et al (2018) Diagnosis-to-treatment interval is an important clinical factor in newly diagnosed diffuse large B-cell lymphoma and has implication for Bias in clinical trials. J Clin Oncol 36:1603–1610
Kochenderfer JN, Sommerville RPT, Lu T et al (2017) Lymphoma remissions caused by anti-CD19 chimeric antigen receptor T cells are associated with high serum interleukin-15 levels. J Clin Oncol 35(16):1803–1813
Abramson J, Gordon L, Palomba ML et al (2018) Updated safety and long-term clinical outcomes in TRANSCEND NHL 001, pivotal trial of lisocabtagen maraleucel (JCAR017) in R/R aggressive NHL. J Clin Oncol 36(15suppl):7505
Swerdlow SH, Campo E, Harris NL et al (2008) WHO classification of tumours of haematopoietic and lymphoid tissues. In: International Agency for Research on Cancer (IARC), 4th edn. Lyon
Hans CP, Weisenburger DD, Greiner TC et al (2004) Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood 103:275–282
Carbone PP, Kaplan HS, Musshoff K, Smithers DW, Tubiana M (1971) Report of the committee on no Hodgkin’s disease staging classification. Cancer Res 31:1860–1861
Sykorova A, Belada D, Smolej L et al (2010) Staging of non-Hodgkin’s lymphoma –recommendations of the Czech Lymphoma Study Group. Klin Onkol 23:146–154
Cheson BD, Horning SJ, Coiffier B et al (1999) Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. NCI Sponsored International Working Group. J Clin Oncol 17(4):1244
Cheson BD, Pfistner B, Juweid ME et al (2007) International Harmonization Project on lymphoma. Revised response criteria for malignant lymphoma. J Clin Oncol 25(5):579–586
Shi Q, Schmitz N, Ou FS, Dixon JG, Cunningham D, Pfreundschuh M, Seymour JF, Jaeger U, Habermann TM, Haioun C, Tilly H, Ghesquieres H, Merli F, Ziepert M, Herbrecht R, Flament J, Fu T, Coiffier B, Flowers CR (2018) Progression-free survival as a surrogate end point for overall survival in first-line diffuse large B-cell lymphoma: an individual patient-level analysis of multiple randomized trials (SEAL). J Clin Oncol 36:2593–2602
Crump M, Neelapu SS, Farooq U, van den Neste E, Kuruvilla J, Westin J, Link BK, Hay A, Cerhan JR, Zhu L, Boussetta S, Feng L, Maurer MJ, Navale L, Wiezorek J, Go WY, Gisselbrecht C (2017) Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study. Blood 130:1800–1808
Blunt DN, Smyth L, Gatov E, Nagamuthu C, Buckstein R, Croxford R, Cheung M (2018) Diagnosis to treatment interval in DLBCL is predictive of overal survival in a large, population-based registry. Blood 132:1700
Yelland LN, Kahan BC, Dent E, Lee KJ, Voysey M, Forbes AB, Cook JA (2018) Prevalence and reporting of recruitment, randomisation and treatment errors in clinical trials: a systematic review. Clin Trials 15(3):278–285
Fatola T, Rutherford SC, Allan JN, Ruan J, Furman RR, Zhengming C, Garcia A, Kong C, Bhavsar EB, Falsenfeld J, Leonard JP, Martin P (2018) Understanding the differences between clinical trial participants and real-world patients with non-Hodgkin lymphoma. Blood 132:2970
Maurer MJ, Farooq U, Wahlen MM et al (2018) Short time between progression after immunochemotherapy and initiation of salvage therapy (PTI) is associated with inferior long-term outcomes in patients with relapsed/refractory DLBCL. ASH Annual Meeting; Abstract No 4204
Harrysson S, Eloranta S, Ekberg S, Enblad G, Jerkeman M, Hasselblom S, Wahlin BE, Andersson PO, Smedby KE (2018) Incidence and outcome of relapsed/refractory diffuse large B-cell lymphoma (DLBCL) in a population-based cohort of 3165 patients in Sweden. Blood 132:2975
Galaznik A, Way N (2018) Patient characteristics and treatment paterns in the first-line and second-line treatment of diffuse large B-cell lymphoma and follicular lymphoma in the United Kingdom, France, and Germany. Blood 132:4232
Locke FL, Ghobaldi A, Jacobskon CA et al (2019) Long-term safety and activity of axicabtabene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1-2 trial. Lancet Oncol 20:31–42
Neelapu SS, Locke FL, Bertlett et al (2017) Axicabtagene Ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med 377:2531–2544
Schuster SJ, Bishop M, Tam C, Waller EK, Borchmann P, McGuirk J, Jäger U, Jaglowski S, Andreadis C, Westin JR, Fleury I, Bachanova V, Foley SR, Ho PJ, Mielke S, Magenau JM, Holte H, Pantano S, Pacaud LB, Awasthi R, Chu J, Anak Ö, Salles G, Maziarz RT, JULIET Investigators (2019) Tisagenlecleucel in adult relapsed and refractory diffuse large B-cell lymphoma. N Engl J Med 380:45–56
Veldman-Jones MH, Lai Z, Wappett M, Harbron CG, Barrett JC, Harrington EA, Thress KS (2015) Reproducible, quantitative, and flexible molecular subtyping of clinical DLBCL samples using the nanostring Countersystem. Clin Cancer Res 21:2367–2378
Greenawalt D, Liang WS, Saif S et al (2017) Comparative analysis of primary versus relapse/refractory DLBCL identifies shifts in mutation spectrum. Oncotarget 8:99237–99244
Funding
This work was supported by research grant AZV 16-31092A, on behalf of the Czech Lymphoma Study Group.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Author J. A. declares that she has no conflict of interest. Author M. J. declares that he has no conflict of interest. Author B. Z. declares that he has no conflict of interest. Author C. R. declares that she has no conflict of interest. Author C. V. declares that he has no conflict of interest. Author K. N. declares that she has no conflict of interest. Author K. P. declares that he has no conflict of interest. Author B. K. declares that she has no conflict of interest. Author H. J. declares that she has no conflict of interest. Author B. D. has received consulting honoraria from Roche, Takeda, Gilead Sciences, Janssen-Cilag, and Debiopharm. Author P. V. has received speaker honoraria from Roche and Gilead Sciences, and consulting for Roche and Takeda. Author P. R. declares that he has no conflict of interest. Author P. J. declares that he has no conflict of interest. Author D. J. declares that he has no conflict of interest. Author M. H. declares that she has no conflict of interest. Author T. M. has received speaker honoraria from Janssen, Gilead Sciences, Takeda, Bristol-Myers-Squibb, Amgen, Abbvie, Roche, Morphosys, Incyte. Consulting and Advisory for Takeda, Bristol-Myers-Squibb, Incyte, Abbvie, Amgen, Roche, Gilead Sciences, Janssen, Celgene, Morphosys.
Ethical approval
This retrospective chart review study involving human participants was in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 73.7 kb)
Rights and permissions
About this article
Cite this article
Janikova, A., Michalka, J., Bortlicek, Z. et al. The interval between progression and therapy initiation is the key prognostic parameter in relapsing diffuse large B cell lymphoma: analysis from the Czech Lymphoma Study Group database (NIHIL). Ann Hematol 99, 1583–1594 (2020). https://doi.org/10.1007/s00277-020-04099-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-020-04099-y