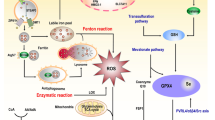
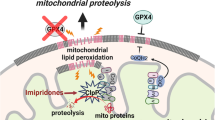
Abstract
Ferroptosis is a form of cell death that is regulated by iron and characterized by the buildup of lipid peroxides (LPO) and subsequent rupture of the cell membrane. The molecular mechanisms of ferroptosis involve metabolic pathways related to iron, lipids, and amino acids, which contribute to the production of lipid reactive oxygen species (ROS). In recent years, there has been increasing attention on the occurrence of ferroptosis in various diseases. Ferroptosis has been found to play a crucial role in cardiovascular diseases, digestive diseases, respiratory and immunological diseases, and particularly in malignancies. However, there is still a lack of studies on ferroptosis in acute myeloid leukemia (AML). This paper provides a comprehensive review of the mechanism of ferroptosis and its regulatory molecules and therapeutic agents in AML. It also evaluates the relationship between ferroptosis-related genes (FRGs), non-coding RNAs (ncRNAs), and prognosis to develop prognostic molecular models in AML. The study also explores the association between ferroptosis and immune infiltration in AML, to identify novel potential target regimens for AML.




Similar content being viewed by others
Data availability
All data generated or analysed during this study are included in this published article.
References
Kroemer G, Galluzzi L, Vandenabeele P, Abrams J, Alnemri ES, Baehrecke EH et al (2009) Classification of cell death: recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ 16:3–11. https://doi.org/10.1038/cdd.2008.150
D’Souza CA, Heitman J (2001) Dismantling the Cryptococcus coat. Trends Microbiol 9:112–113. https://doi.org/10.1016/s0966-842x(00)01945-4
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE et al (2012) Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149:1060–1072. https://doi.org/10.1016/j.cell.2012.03.042
Stockwell BR, Friedmann Angeli JP, Bayir H, Bush AI, Conrad M, Dixon SJ et al (2017) Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell 171:273–285. https://doi.org/10.1016/j.cell.2017.09.021
Maimaitizunong R, Wang K, Li H (2022) Ferroptosis and its emerging role in esophageal cancer. Front Mol Biosci 9:1027912. https://doi.org/10.3389/fmolb.2022.1027912
Lei G, Zhuang L, Gan B (2022) Targeting ferroptosis as a vulnerability in cancer. Nat Rev Cancer 22:381–396. https://doi.org/10.1038/s41568-022-00459-0
Yang WS, SriRamaratnam R, Welsch ME, Shimada K, Skouta R, Viswanathan VS et al (2014) Regulation of ferroptotic cancer cell death by GPX4. Cell 156:317–331. https://doi.org/10.1016/j.cell.2013.12.010
Bersuker K, Hendricks JM, Li Z, Magtanong L, Ford B, Tang PH et al (2019) The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature 575:688–692. https://doi.org/10.1038/s41586-019-1705-2
Doll S, Freitas FP, Shah R, Aldrovandi M, da Silva MC, Ingold I et al (2019) FSP1 is a glutathione-independent ferroptosis suppressor. Nature 575:693–698. https://doi.org/10.1038/s41586-019-1707-0
Mao C, Liu X, Zhang Y, Lei G, Yan Y, Lee H et al (2021) DHODH-mediated ferroptosis defence is a targetable vulnerability in cancer. Nature 593:586–590. https://doi.org/10.1038/s41586-021-03539-7
Xie Y, Hou W, Song X, Yu Y, Huang J, Sun X et al (2016) Ferroptosis: process and function. Cell Death Differ 23:369–379. https://doi.org/10.1038/cdd.2015.158
Valashedi MR, Nikoo A, Najafi-Ghalehlou N, Tomita K, Kuwahara Y, Sato T et al (2022) Pharmacological targeting of ferroptosis in cancer treatment. Curr Cancer Drug Targets 22:108–125. https://doi.org/10.2174/1568009621666211202091523
Dixon SJ, Stockwell BR (2014) The role of iron and reactive oxygen species in cell death. Nat Chem Biol 10:9–17. https://doi.org/10.1038/nchembio.1416
Yagoda N, von Rechenberg M, Zaganjor E, Bauer AJ, Yang WS, Fridman DJ et al (2007) RAS-RAF-MEK-dependent oxidative cell death involving voltage-dependent anion channels. Nature 447:864–868. https://doi.org/10.1038/nature05859
Yu Y, Xie Y, Cao L, Yang L, Yang M, Lotze MT et al (2015) The ferroptosis inducer erastin enhances sensitivity of acute myeloid leukemia cells to chemotherapeutic agents. Mol Cell Oncol 2:e1054549. https://doi.org/10.1080/23723556.2015.1054549
Du J, Wang T, Li Y, Zhou Y, Wang X, Yu X et al (2019) DHA inhibits proliferation and induces ferroptosis of leukemia cells through autophagy dependent degradation of ferritin. Free Radic Biol Med 131:356–369. https://doi.org/10.1016/j.freeradbiomed.2018.12.011
Wang HT, Ju J, Wang SC, Zhang YH, Liu CY, Wang T et al (2022) Insights into ferroptosis, a novel target for the therapy of cancer. Front Oncol 12:812534. https://doi.org/10.3389/fonc.2022.812534
Torti SV, Manz DH, Paul BT, Blanchette-Farra N, Torti FM (2018) Iron and cancer. Annu Rev Nutr 38:97–125. https://doi.org/10.1146/annurev-nutr-082117-051732
Cheng Y, Zak O, Aisen P, Harrison SC, Walz T (2004) Structure of the human transferrin receptor-transferrin complex. Cell 116:565–576. https://doi.org/10.1016/s0092-8674(04)00130-8
Kazan HH, Urfali-Mamatoglu C, Gunduz U (2017) Iron metabolism and drug resistance in cancer. Biometals 30:629–641. https://doi.org/10.1007/s10534-017-0037-7
Yang WS, Stockwell BR (2008) Synthetic lethal screening identifies compounds activating iron-dependent, nonapoptotic cell death in oncogenic-RAS-harboring cancer cells. Chem Biol 15:234–245. https://doi.org/10.1016/j.chembiol.2008.02.010
Alvarez SW, Sviderskiy VO, Terzi EM, Papagiannakopoulos T, Moreira AL, Adams S et al (2017) NFS1 undergoes positive selection in lung tumours and protects cells from ferroptosis. Nature 551:639–643. https://doi.org/10.1038/nature24637
Torti SV, Torti FM (2013) Iron and cancer: more ore to be mined. Nat Rev Cancer 13:342–355. https://doi.org/10.1038/nrc3495
Yanatori I, Kishi F (2019) DMT1 and iron transport. Free Radic Biol Med 133:55–63. https://doi.org/10.1016/j.freeradbiomed.2018.07.020
Harrison PM, Arosio P (1996) The ferritins: molecular properties, iron storage function and cellular regulation. Biochim Biophys Acta 1275:161–203. https://doi.org/10.1016/0005-2728(96)00022-9
Shen Z, Liu T, Li Y, Lau J, Yang Z, Fan W et al (2018) Fenton-reaction-acceleratable magnetic nanoparticles for ferroptosis therapy of orthotopic brain tumors. ACS Nano 12:11355–11365. https://doi.org/10.1021/acsnano.8b06201
Patel SJ, Frey AG, Palenchar DJ, Achar S, Bullough KZ, Vashisht A et al (2019) A PCBP1-BolA2 chaperone complex delivers iron for cytosolic [2Fe-2S] cluster assembly. Nat Chem Biol 15:872–881. https://doi.org/10.1038/s41589-019-0330-6
Santana-Codina N, Mancias JD (2018) The role of NCOA4-mediated ferritinophagy in health and disease. Pharmaceuticals (Basel) 11. https://doi.org/10.3390/ph11040114
Mancias JD, Pontano Vaites L, Nissim S, Biancur DE, Kim AJ, Wang X et al (2015) Ferritinophagy via NCOA4 is required for erythropoiesis and is regulated by iron dependent HERC2-mediated proteolysis. Elife 4. https://doi.org/10.7554/eLife.10308
Kremer DM, Nelson BS, Lin L, Yarosz EL, Halbrook CJ, Kerk SA et al (2021) GOT1 inhibition promotes pancreatic cancer cell death by ferroptosis. Nat Commun 12:4860. https://doi.org/10.1038/s41467-021-24859-2
Chang LC, Chiang SK, Chen SE, Yu YL, Chou RH, Chang WC (2018) Heme oxygenase-1 mediates BAY 11–7085 induced ferroptosis. Cancer Lett 416:124–137. https://doi.org/10.1016/j.canlet.2017.12.025
Valashedi MR, Bamshad C, Najafi-Ghalehlou N, Nikoo A, Tomita K, Kuwahara Y et al (2022) Non-coding RNAs in ferroptotic cancer cell death pathway: meet the new masters. Hum Cell 35:972–994. https://doi.org/10.1007/s13577-022-00699-0
Bridges R, Lutgen V, Lobner D, Baker DA (2012) Thinking outside the cleft to understand synaptic activity: contribution of the cystine-glutamate antiporter (System xc-) to normal and pathological glutamatergic signaling. Pharmacol Rev 64:780–802. https://doi.org/10.1124/pr.110.003889
Koppula P, Zhang Y, Zhuang L, Gan B (2018) Amino acid transporter SLC7A11/xCT at the crossroads of regulating redox homeostasis and nutrient dependency of cancer. Cancer Commun (Lond) 38:12. https://doi.org/10.1186/s40880-018-0288-x
Zhao X, Zhou M, Yang Y, Luo M (2021) The ubiquitin hydrolase OTUB1 promotes glioma cell stemness via suppressing ferroptosis through stabilizing SLC7A11 protein. Bioengineered 12:12636–12645. https://doi.org/10.1080/21655979.2021.2011633
Liu X, Zhang Y, Zhuang L, Olszewski K, Gan B (2021) NADPH debt drives redox bankruptcy: SLC7A11/xCT-mediated cystine uptake as a double-edged sword in cellular redox regulation. Genes Dis 8:731–745. https://doi.org/10.1016/j.gendis.2020.11.010
Dixon SJ, Patel DN, Welsch M, Skouta R, Lee ED, Hayano M et al (2014) Pharmacological inhibition of cystine-glutamate exchange induces endoplasmic reticulum stress and ferroptosis. Elife 3:e02523. https://doi.org/10.7554/eLife.02523
Brigelius-Flohé R, Maiorino M (2013) Glutathione peroxidases. Biochim Biophys Acta 1830:3289–3303. https://doi.org/10.1016/j.bbagen.2012.11.020
Kagan VE, Mao G, Qu F, Angeli JP, Doll S, Croix CS et al (2017) Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat Chem Biol 13:81–90. https://doi.org/10.1038/nchembio.2238
Magtanong L, Dixon SJ (2018) Ferroptosis and brain injury. Dev Neurosci 40:382–395. https://doi.org/10.1159/000496922
Wei Y, Lv H, Shaikh AB, Han W, Hou H, Zhang Z et al (2020) Directly targeting glutathione peroxidase 4 may be more effective than disrupting glutathione on ferroptosis-based cancer therapy. Biochim Biophys Acta Gen Subj 1864:129539. https://doi.org/10.1016/j.bbagen.2020.129539
Brigelius-Flohé R, Flohé L (2020) Regulatory phenomena in the glutathione peroxidase superfamily. Antioxid Redox Signal 33:498–516. https://doi.org/10.1089/ars.2019.7905
Shimada K, Skouta R, Kaplan A, Yang WS, Hayano M, Dixon SJ et al (2016) Global survey of cell death mechanisms reveals metabolic regulation of ferroptosis. Nat Chem Biol 12:497–503. https://doi.org/10.1038/nchembio.2079
Gaschler MM, Andia AA, Liu H, Csuka JM, Hurlocker B, Vaiana CA et al (2018) FINO(2) initiates ferroptosis through GPX4 inactivation and iron oxidation. Nat Chem Biol 14:507–515. https://doi.org/10.1038/s41589-018-0031-6
Jiang L, Kon N, Li T, Wang SJ, Su T, Hibshoosh H et al (2015) Ferroptosis as a p53-mediated activity during tumour suppression. Nature 520:57–62. https://doi.org/10.1038/nature14344
Lo M, Ling V, Low C, Wang YZ, Gout PW (2010) Potential use of the anti-inflammatory drug, sulfasalazine, for targeted therapy of pancreatic cancer. Curr Oncol 17:9–16. https://doi.org/10.3747/co.v17i3.485
Cramer SL, Saha A, Liu J, Tadi S, Tiziani S, Yan W et al (2017) Systemic depletion of L-cyst(e)ine with cyst(e)inase increases reactive oxygen species and suppresses tumor growth. Nat Med 23:120–127. https://doi.org/10.1038/nm.4232
Lőrincz T, Jemnitz K, Kardon T, Mandl J, Szarka A (2015) Ferroptosis is involved in acetaminophen induced cell death. Pathol Oncol Res 21:1115–1121. https://doi.org/10.1007/s12253-015-9946-3
Liang Z, Zhao W, Li X, Wang L, Meng L, Yu R (2021) Cisplatin synergizes with PRLX93936 to induce ferroptosis in non-small cell lung cancer cells. Biochem Biophys Res Commun 569:79–85. https://doi.org/10.1016/j.bbrc.2021.06.088
Dolma S, Lessnick SL, Hahn WC, Stockwell BR (2003) Identification of genotype-selective antitumor agents using synthetic lethal chemical screening in engineered human tumor cells. Cancer Cell 3:285–296. https://doi.org/10.1016/s1535-6108(03)00050-3
Woo JH, Shimoni Y, Yang WS, Subramaniam P, Iyer A, Nicoletti P et al (2015) Elucidating compound mechanism of action by network perturbation analysis. Cell 162:441–451. https://doi.org/10.1016/j.cell.2015.05.056
Hassannia B, Wiernicki B, Ingold I, Qu F, Van Herck S, Tyurina YY et al (2018) Nano-targeted induction of dual ferroptotic mechanisms eradicates high-risk neuroblastoma. J Clin Invest 128:3341–3355. https://doi.org/10.1172/jci99032
Shin D, Kim EH, Lee J, Roh JL (2018) Nrf2 inhibition reverses resistance to GPX4 inhibitor-induced ferroptosis in head and neck cancer. Free Radic Biol Med 129:454–462. https://doi.org/10.1016/j.freeradbiomed.2018.10.426
Sun X, Ou Z, Chen R, Niu X, Chen D, Kang R et al (2016) Activation of the p62-Keap1-NRF2 pathway protects against ferroptosis in hepatocellular carcinoma cells. Hepatology 63:173–184. https://doi.org/10.1002/hep.28251
Eling N, Reuter L, Hazin J, Hamacher-Brady A, Brady NR (2015) Identification of artesunate as a specific activator of ferroptosis in pancreatic cancer cells. Oncoscience 2:517–532. https://doi.org/10.18632/oncoscience.160
Basit F, van Oppen LM, Schöckel L, Bossenbroek HM, van Emst-de Vries SE, Hermeling JC et al (2017) Mitochondrial complex I inhibition triggers a mitophagy-dependent ROS increase leading to necroptosis and ferroptosis in melanoma cells. Cell Death Dis 8:e2716. https://doi.org/10.1038/cddis.2017.133
Dietrich C, Hofmann TG (2021) Ferroptosis meets cell-cell contacts. Cells 10. https://doi.org/10.3390/cells10092462
Mou Y, Wang J, Wu J, He D, Zhang C, Duan C et al (2019) Ferroptosis, a new form of cell death: opportunities and challenges in cancer. J Hematol Oncol 12:34. https://doi.org/10.1186/s13045-019-0720-y
Dixon SJ, Winter GE, Musavi LS, Lee ED, Snijder B, Rebsamen M et al (2015) Human haploid cell genetics reveals roles for lipid metabolism genes in nonapoptotic cell death. ACS Chem Biol 10:1604–1609. https://doi.org/10.1021/acschembio.5b00245
Cui Y, Zhang Y, Zhao X, Shao L, Liu G, Sun C et al (2021) ACSL4 exacerbates ischemic stroke by promoting ferroptosis-induced brain injury and neuroinflammation. Brain Behav Immun 93:312–321. https://doi.org/10.1016/j.bbi.2021.01.003
Ayala A, Muñoz MF, Argüelles S (2014) Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev 2014:360438. https://doi.org/10.1155/2014/360438
Angeli JPF, Shah R, Pratt DA, Conrad M (2017) Ferroptosis inhibition: mechanisms and opportunities. Trends Pharmacol Sci 38:489–498. https://doi.org/10.1016/j.tips.2017.02.005
Wu M, Xu LG, Li X, Zhai Z, Shu HB (2002) AMID, an apoptosis-inducing factor-homologous mitochondrion-associated protein, induces caspase-independent apoptosis. J Biol Chem 277:25617–25623. https://doi.org/10.1074/jbc.M202285200
Vasan K, Werner M, Chandel NS (2020) Mitochondrial metabolism as a target for cancer therapy. Cell Metab 32:341–352. https://doi.org/10.1016/j.cmet.2020.06.019
Martínez-Reyes I, Cardona LR, Kong H, Vasan K, McElroy GS, Werner M et al (2020) Mitochondrial ubiquinol oxidation is necessary for tumour growth. Nature 585:288–292. https://doi.org/10.1038/s41586-020-2475-6
Chen SF, Ruben RL, Dexter DL (1986) Mechanism of action of the novel anticancer agent 6-fluoro-2-(2′-fluoro-1,1′-biphenyl-4-yl)-3-methyl-4-quinolinecarbo xylic acid sodium salt (NSC 368390): inhibition of de novo pyrimidine nucleotide biosynthesis. Cancer Res 46:5014–5019
Urba S, Doroshow J, Cripps C, Robert F, Velez-Garcia E, Dallaire B et al (1992) Multicenter phase II trial of brequinar sodium in patients with advanced squamous-cell carcinoma of the head and neck. Cancer Chemother Pharmacol 31:167–169. https://doi.org/10.1007/bf00685106
Soula M, Weber RA, Zilka O, Alwaseem H, La K, Yen F et al (2020) Metabolic determinants of cancer cell sensitivity to canonical ferroptosis inducers. Nat Chem Biol 16:1351–1360. https://doi.org/10.1038/s41589-020-0613-y
Vasquez-Vivar J, Shi Z, Tan S (2022) Tetrahydrobiopterin in cell function and death mechanisms. Antioxid Redox Signal 37:171–183. https://doi.org/10.1089/ars.2021.0136
Kraft VAN, Bezjian CT, Pfeiffer S, Ringelstetter L, Müller C, Zandkarimi F et al (2020) GTP cyclohydrolase 1/tetrahydrobiopterin counteract ferroptosis through lipid remodeling. ACS Cent Sci 6:41–53. https://doi.org/10.1021/acscentsci.9b01063
Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel JD et al (1999) Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev 13:76–86. https://doi.org/10.1101/gad.13.1.76
Valashedi MR, Najafi-Ghalehlou N, Nikoo A, Bamshad C, Tomita K, Kuwahara Y et al (2021) Cashing in on ferroptosis against tumor cells: usher in the next chapter. Life Sci 285:119958. https://doi.org/10.1016/j.lfs.2021.119958
Lu K, Alcivar AL, Ma J, Foo TK, Zywea S, Mahdi A et al (2017) NRF2 Induction supporting breast cancer cell survival is enabled by oxidative stress-induced DPP3-KEAP1 interaction. Cancer Res 77:2881–2892. https://doi.org/10.1158/0008-5472.Can-16-2204
Zhang DD (2006) Mechanistic studies of the Nrf2-Keap1 signaling pathway. Drug Metab Rev 38:769–789. https://doi.org/10.1080/03602530600971974
Chen D, Tavana O, Chu B, Erber L, Chen Y, Baer R et al (2017) NRF2 is a major target of ARF in p53-independent tumor suppression. Mol Cell 68:224-232.e224. https://doi.org/10.1016/j.molcel.2017.09.009
Kang R, Kroemer G, Tang D (2019) The tumor suppressor protein p53 and the ferroptosis network. Free Radic Biol Med 133:162–168. https://doi.org/10.1016/j.freeradbiomed.2018.05.074
Ou Y, Wang SJ, Li D, Chu B, Gu W (2016) Activation of SAT1 engages polyamine metabolism with p53-mediated ferroptotic responses. Proc Natl Acad Sci U S A 113:E6806-e6812. https://doi.org/10.1073/pnas.1607152113
Xie Y, Zhu S, Song X, Sun X, Fan Y, Liu J et al (2017) The tumor suppressor p53 limits ferroptosis by blocking DPP4 activity. Cell Rep 20:1692–1704. https://doi.org/10.1016/j.celrep.2017.07.055
Tarangelo A, Magtanong L, Bieging-Rolett KT, Li Y, Ye J, Attardi LD et al (2018) p53 suppresses metabolic stress-induced ferroptosis in cancer cells. Cell Rep 22:569–575. https://doi.org/10.1016/j.celrep.2017.12.077
Lan H, Gao Y, Zhao Z, Mei Z, Wang F (2022) Ferroptosis: redox imbalance and hematological tumorigenesis. Front Oncol 12:834681. https://doi.org/10.3389/fonc.2022.834681
Daver NG, Maiti A, Kadia TM, Vyas P, Majeti R, Wei AH et al (2022) TP53-mutated myelodysplastic syndrome and acute myeloid leukemia: biology, current therapy, and future directions. Cancer Discov 12:2516–2529. https://doi.org/10.1158/2159-8290.Cd-22-0332
Ley TJ, Miller C, Ding L, Raphael BJ, Mungall AJ, Robertson A et al (2013) Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med 368:2059–2074. https://doi.org/10.1056/NEJMoa1301689
Zhu HY, Huang ZX, Chen GQ, Sheng F, Zheng YS (2019) Typhaneoside prevents acute myeloid leukemia (AML) through suppressing proliferation and inducing ferroptosis associated with autophagy. Biochem Biophys Res Commun 516:1265–1271. https://doi.org/10.1016/j.bbrc.2019.06.070
Du Y, Bao J, Zhang MJ, Li LL, Xu XL, Chen H et al (2020) Targeting ferroptosis contributes to ATPR-induced AML differentiation via ROS-autophagy-lysosomal pathway. Gene 755:144889. https://doi.org/10.1016/j.gene.2020.144889
Greco G, Schnekenburger M, Catanzaro E, Turrini E, Ferrini F, Sestili P et al (2021) Discovery of sulforaphane as an inducer of ferroptosis in U-937 leukemia cells: expanding its anticancer potential. Cancers (Basel) 14. https://doi.org/10.3390/cancers14010076
Birsen R, Larrue C, Decroocq J, Johnson N, Guiraud N, Gotanegre M et al (2022) APR-246 induces early cell death by ferroptosis in acute myeloid leukemia. Haematologica 107:403–416. https://doi.org/10.3324/haematol.2020.259531
Lai X, Sun Y, Zhang X, Wang D, Wang J, Wang H et al (2022) Honokiol induces ferroptosis by upregulating HMOX1 in acute myeloid leukemia cells. Front Pharmacol 13:897791. https://doi.org/10.3389/fphar.2022.897791
Ma H, Liu Y, Miao Z, Cheng S, Zhu Y, Wu Y et al (2022) Neratinib inhibits proliferation and promotes apoptosis of acute myeloid leukemia cells by activating autophagy-dependent ferroptosis. Drug Dev Res 83:1641–1653. https://doi.org/10.1002/ddr.21983
Pardieu B, Pasanisi J, Ling F, Dal Bello R, Penneroux J, Su A et al (2022) Cystine uptake inhibition potentiates front-line therapies in acute myeloid leukemia. Leukemia 36:1585–1595. https://doi.org/10.1038/s41375-022-01573-6
Lv Q, Niu H, Yue L, Liu J, Yang L, Liu C et al (2020) Abnormal ferroptosis in myelodysplastic syndrome. Front Oncol 10:1656. https://doi.org/10.3389/fonc.2020.01656
Battipaglia G, Massoud R, Ahmed SO, Legrand O, El Cheikh J, Youniss R et al (2019) Efficacy and feasibility of sorafenib as a maintenance agent after allogeneic hematopoietic stem cell transplantation for Fms-like Tyrosine kinase 3 mutated acute myeloid leukemia: an update. Clin Lymphoma Myeloma Leuk 19:506–508. https://doi.org/10.1016/j.clml.2019.04.004
Maslah N, Salomao N, Drevon L, Verger E, Partouche N, Ly P et al (2020) Synergistic effects of PRIMA-1(Met) (APR-246) and 5-azacitidine in TP53-mutated myelodysplastic syndromes and acute myeloid leukemia. Haematologica 105:1539–1551. https://doi.org/10.3324/haematol.2019.218453
Dong LH, Huang JJ, Zu P, Liu J, Gao X, Du JW et al (2021) CircKDM4C upregulates P53 by sponging hsa-let-7b-5p to induce ferroptosis in acute myeloid leukemia. Environ Toxicol 36:1288–1302. https://doi.org/10.1002/tox.23126
Wang F, Lv H, Zhao B, Zhou L, Wang S, Luo J et al (2019) Iron and leukemia: new insights for future treatments. J Exp Clin Cancer Res 38:406. https://doi.org/10.1186/s13046-019-1397-3
Callens C, Coulon S, Naudin J, Radford-Weiss I, Boissel N, Raffoux E et al (2010) Targeting iron homeostasis induces cellular differentiation and synergizes with differentiating agents in acute myeloid leukemia. J Exp Med 207:731–750. https://doi.org/10.1084/jem.20091488
Lotze MT, Tracey KJ (2005) High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol 5:331–342. https://doi.org/10.1038/nri1594
Ye F, Chai W, Xie M, Yang M, Yu Y, Cao L et al (2019) HMGB1 regulates erastin-induced ferroptosis via RAS-JNK/p38 signaling in HL-60/NRAS(Q61L) cells. Am J Cancer Res 9:730–739
Liu MZ, Kong N, Zhang GY, Xu Q, Xu Y, Ke P et al (2022) The critical role of ferritinophagy in human disease. Front Pharmacol 13:933732. https://doi.org/10.3389/fphar.2022.933732
Gao M, Monian P, Pan Q, Zhang W, Xiang J, Jiang X (2016) Ferroptosis is an autophagic cell death process. Cell Res 26:1021–1032. https://doi.org/10.1038/cr.2016.95
Qin G, Zhao C, Zhang L, Liu H, Quan Y, Chai L et al (2015) Dihydroartemisinin induces apoptosis preferentially via a Bim-mediated intrinsic pathway in hepatocarcinoma cells. Apoptosis 20:1072–1086. https://doi.org/10.1007/s10495-015-1132-2
Grignano E, Cantero-Aguilar L, Tuerdi Z, Chabane T, Vazquez R, Johnson N et al (2023) Dihydroartemisinin-induced ferroptosis in acute myeloid leukemia: links to iron metabolism and metallothionein. Cell Death Discov 9:97. https://doi.org/10.1038/s41420-023-01371-8
Yusuf RZ, Saez B, Sharda A, van Gastel N, Yu VWC, Baryawno N et al (2020) Aldehyde dehydrogenase 3a2 protects AML cells from oxidative death and the synthetic lethality of ferroptosis inducers. Blood 136:1303–1316. https://doi.org/10.1182/blood.2019001808
Papaemmanuil E, Gerstung M, Bullinger L, Gaidzik VI, Paschka P, Roberts ND et al (2016) Genomic classification and prognosis in acute myeloid leukemia. N Engl J Med 374:2209–2221. https://doi.org/10.1056/NEJMoa1516192
Sabatier M, Birsen R, Lauture L, Mouche S, Angelino P, Dehairs J et al (2023) C/EBPa confers dependence to fatty acid anabolic pathways and vulnerability to lipid oxidative stress-induced ferroptosis in FLT3-mutant leukemia. Cancer Discov. https://doi.org/10.1158/2159-8290.Cd-22-0411
Long F, Lin Z, Long Q, Lu Z, Zhu K, Zhao M et al (2023) CircZBTB46 Protects acute myeloid leukemia cells from ferroptotic cell death by upregulating SCD. Cancers (Basel) 15. https://doi.org/10.3390/cancers15020459
Takebe G, Yarimizu J, Saito Y, Hayashi T, Nakamura H, Yodoi J et al (2002) A comparative study on the hydroperoxide and thiol specificity of the glutathione peroxidase family and selenoprotein P. J Biol Chem 277:41254–41258. https://doi.org/10.1074/jbc.M202773200
Wei J, Xie Q, Liu X, Wan C, Wu W, Fang K et al (2020) Identification the prognostic value of glutathione peroxidases expression levels in acute myeloid leukemia. Ann Transl Med 8:678. https://doi.org/10.21037/atm-20-3296
Yin Z, Li F, Zhou Q, Zhu J, Liu Z, Huang J et al (2022) A ferroptosis-related gene signature and immune infiltration patterns predict the overall survival in acute myeloid leukemia patients. Front Mol Biosci 9:959738. https://doi.org/10.3389/fmolb.2022.959738
Wei J, Nai GY, Dai Y, Huang XJ, Xiong MY, Yao XY et al (2021) Dipetidyl peptidase-4 and transferrin receptor serve as prognostic biomarkers for acute myeloid leukemia. Ann Transl Med 9:1381. https://doi.org/10.21037/atm-21-3368
Chen Y, Peng W, Zhang Z, Liu X, Yang P, Fu C et al (2023) The relationship between MUC19 copy number variation and growth traits of Chinese cattle. Gene 851:147010. https://doi.org/10.1016/j.gene.2022.147010
Han C, Zheng J, Li F, Guo W, Cai C (2022) Novel prognostic signature for acute myeloid leukemia: bioinformatics analysis of combined CNV-driven and ferroptosis-related genes. Front Genet 13:849437. https://doi.org/10.3389/fgene.2022.849437
Carver JA, Rekas A, Thorn DC, Wilson MR (2003) Small heat-shock proteins and clusterin: intra- and extracellular molecular chaperones with a common mechanism of action and function? IUBMB Life 55:661–668. https://doi.org/10.1080/15216540310001640498
Zheng Z, Hong X, Huang X, Jiang X, Jiang H, Huang Y et al (2022) Comprehensive analysis of ferroptosis-related gene signatures as a potential therapeutic target for acute myeloid leukemia: a bioinformatics analysis and experimental verification. Front Oncol 12:930654. https://doi.org/10.3389/fonc.2022.930654
Zheng Z, Wu W, Lin Z, Liu S, Chen Q, Jiang X et al (2021) Identification of seven novel ferroptosis-related long non-coding RNA signatures as a diagnostic biomarker for acute myeloid leukemia. BMC Med Genomics 14:236. https://doi.org/10.1186/s12920-021-01085-9
Tao Y, Wei L, You H (2022) Ferroptosis-related gene signature predicts the clinical outcome in pediatric acute myeloid leukemia patients and refines the 2017 ELN classification system. Front Mol Biosci 9:954524. https://doi.org/10.3389/fmolb.2022.954524
Wang W, Green M, Choi JE, Gijón M, Kennedy PD, Johnson JK et al (2019) CD8(+) T cells regulate tumour ferroptosis during cancer immunotherapy. Nature 569:270–274. https://doi.org/10.1038/s41586-019-1170-y
Liao P, Wang W, Wang W, Kryczek I, Li X, Bian Y et al (2022) CD8(+) T cells and fatty acids orchestrate tumor ferroptosis and immunity via ACSL4. Cancer Cell 40:365-378.e366. https://doi.org/10.1016/j.ccell.2022.02.003
Wen Q, Liu J, Kang R, Zhou B, Tang D (2019) The release and activity of HMGB1 in ferroptosis. Biochem Biophys Res Commun 510:278–283. https://doi.org/10.1016/j.bbrc.2019.01.090
Luo X, Gong HB, Gao HY, Wu YP, Sun WY, Li ZQ et al (2021) Oxygenated phosphatidylethanolamine navigates phagocytosis of ferroptotic cells by interacting with TLR2. Cell Death Differ 28:1971–1989. https://doi.org/10.1038/s41418-020-00719-2
Ma X, Xiao L, Liu L, Ye L, Su P, Bi E et al (2021) CD36-mediated ferroptosis dampens intratumoral CD8(+) T cell effector function and impairs their antitumor ability. Cell Metab 33:1001-1012.e1005. https://doi.org/10.1016/j.cmet.2021.02.015
Xu C, Sun S, Johnson T, Qi R, Zhang S, Zhang J et al (2021) The glutathione peroxidase Gpx4 prevents lipid peroxidation and ferroptosis to sustain Treg cell activation and suppression of antitumor immunity. Cell Rep 35:109235. https://doi.org/10.1016/j.celrep.2021.109235
Giuliani C (2019) The flavonoid quercetin induces AP-1 activation in FRTL-5 thyroid cells. Antioxidants (Basel) 8. https://doi.org/10.3390/antiox8050112
Huang X, Zhou D, Ye X, Jin J (2022) A novel ferroptosis-related gene signature can predict prognosis and influence immune microenvironment in acute myeloid leukemia. Bosn J Basic Med Sci 22:608–628. https://doi.org/10.17305/bjbms.2021.6274
Cagnoni AJ, Giribaldi ML, Blidner AG, Cutine AM, Gatto SG, Morales RM et al (2021) Galectin-1 fosters an immunosuppressive microenvironment in colorectal cancer by reprogramming CD8(+) regulatory T cells. Proc Natl Acad Sci U S A 118. https://doi.org/10.1073/pnas.2102950118
Wang J, Zhuo Z, Wang Y, Yang S, Chen J, Wang Y et al (2021) Identification and validation of a prognostic risk-scoring model based on ferroptosis-associated cluster in acute myeloid leukemia. Front Cell Dev Biol 9:800267. https://doi.org/10.3389/fcell.2021.800267
Mishra A, Tamari R, DeZern AE, Byrne MT, Gooptu M, Chen YB et al (2022) Eprenetapopt plus azacitidine after allogeneic hematopoietic stem-cell transplantation for TP53-mutant acute myeloid leukemia and myelodysplastic syndromes. J Clin Oncol 40:3985–3993. https://doi.org/10.1200/jco.22.00181
Cluzeau T, Sebert M, Rahmé R, Cuzzubbo S, Lehmann-Che J, Madelaine I et al (2021) Eprenetapopt plus azacitidine in TP53-mutated myelodysplastic syndromes and acute myeloid leukemia: a phase II study by the Groupe Francophone des Myélodysplasies (GFM). J Clin Oncol 39:1575–1583. https://doi.org/10.1200/jco.20.02342
Acknowledgements
We thank the Adobe illustrator (AI) software for helping us draw figures.
Author information
Authors and Affiliations
Contributions
TX, WY, ZY, and GY contributed to the literature search and conception of the manuscript; TX and WY wrote the manuscript; LB reviewed and revised the manuscript; and all authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tang, X., Wang, Y., Zhu, Y. et al. Basic mechanisms and novel potential therapeutic targets for ferroptosis in acute myeloid leukemia. Ann Hematol 102, 1985–1999 (2023). https://doi.org/10.1007/s00277-023-05293-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-023-05293-4