Abstract
To compare the outcomes of patients with hematological malignancies who received ATG-Fresenius (ATG-F) 20 mg/kg versus those who received ATG-Genzyme (ATG-G) 10 mg/kg in an unrelated donor hematopoietic stem cell transplantation (HSCT) procedure, a total of 186 patients who underwent their first allogeneic HSCT with an unrelated donor were retrospectively analyzed. One hundred and seven patients received ATG-F, and seventy-nine patients received ATG-G. Multivariate analysis showed that the type of ATG preparation had no effect on neutrophil engraftment (P = 0.61), cumulative incidence of relapse (P = 0.092), nonrelapse mortality (P = 0.44), grade II-IV acute graft-versus-host disease (GVHD) (P = 0.47), chronic GVHD (P = 0.29), overall survival (P = 0.795), recurrence-free survival (P = 0.945) or GVHD-free relapse-free survival (P = 0.082). ATG-G was associated with a lower risk of extensive chronic GVHD and a higher risk of cytomegaloviremia (P = 0.01 and HR = 0.41, P < 0.001 and HR = 4.244, respectively). The results of this study suggest that the preparation of rabbit ATG used for unrelated HSCT should be selected based on the incidence of extensive chronic GVHD of each center, and the posttransplant management strategy should be adjusted according to the ATG preparation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Allogeneic hematopoietic stem cell transplantation is an important method to cure hematological malignancies, and unrelated donors are important alternatives for patients without HLA identical sibling donors. Anti-thymocyte globulin (ATG) can decrease the incidence of graft-versus-host disease (GVHD) in HLA-matched and HLA-mismatched unrelated transplantation and improves the survival of mismatched unrelated transplantation [1,2,3,4,5,6,7,8,9,10]. Two commercial preparations of rabbit ATG, ATG-Fresenius (ATG-F, currently sold as ATG-Grafalon) and ATG-Genzyme (ATG-G), are widely used in hematopoietic stem cell transplantation. ATG-G is manufactured by rabbit immunization against human thymocytes, whereas ATG-F is produced by immunizing rabbits with the Jurkat human T-lymphoblastic cell line [11]. The different manufacturing methods result in discrepancies in antibody specificities and immunomodulatory effects independent of their ability to deplete T cells [11,12,13]. Comparisons of their protective role in unrelated transplantation have been conducted in several studies, but the conclusions are varied [14,15,16,17,18]. In two of these studies, the doses of both products were not fixed [14, 15], so the findings are difficult to interpret. The remaining studies compared ATG-F and ATG-G at fixed doses; however, the case number was too small, and multivariate analyses were not conducted [16,17,18]. Therefore, studies are still needed to compare the efficacy of ATG-F and ATG-G at fixed doses. In this study, we retrospectively analyzed 186 unrelated donor transplantation patients from a single center who received ATG-F 20 mg/kg or ATG-G 10 mg/kg in their transplantation procedure and compared the outcomes of patients who received different ATGs.
Patients and methods
Patients
From August 2007 to May 2021, a total of 186 patients diagnosed with malignant hematological diseases without matched sibling donors underwent their first allo-SCT procedure at Xinqiao Hospital, Army Medical University, with an HLA-matched (10/10) or mismatched (9/10 or 8/10) unrelated donor following ATG-containing conditions. All donors were HLA fully matched (10/10) or mismatched at one or two loci (9/10 or 8/10) (HLA-A, B, C, DRB1, DQB1) by high-resolution HLA typing. Patients who previously underwent allogenic transplantation were excluded. This study was approved by the Ethics Committee of Xinqiao Hospital, Army Medical University, and written informed consents were obtained from all patients before transplantation.
Transplantation procedure
The conditioning regimen included TBI/CY, BU/CY, CCNU/MeCCNU + Ara-c + BU + CY (usually used in haploidentical transplantation in China [19]), FB3 or other regimens. For patients who received TBI/CY, 8–9.5 Gy total body irradiation was delivered and fractioned by two days, and a total dose of 120 mg/kg cyclophosphamide was administered. For patients who received BU/CY, a total dose of 12.8 mg/kg intravenous busulfan and 120 mg/kg cyclophosphamide was administered. For patients who received CCNU/MECCNU + Ara-c + BU + CY, 200 mg/m2 lomustine or semustine, a total dose of 8 g/m2 cytarabine, 9.6 mg/kg intravenous busulfan and 3.6 g/m2 cyclophosphamide was administered. For patients who received FB3, a total dose of 150 mg/m2 fludarabine and 390 mg/m2 busulfan was administered. Every patient received a total dose of 10 mg/kg ATG-G or 20 mg/kg ATG-F as part of their conditioning regimen. All patients received unmanipulated granulocyte colony-stimulating factor–mobilized peripheral blood mononuclear cells on day 0 and received cyclosporine/tacrolimus, mycophenolate mofetil and low-dose methotrexate for GVHD prophylaxis. The dose of cyclosporine was adjusted to maintain a trough serum concentration of 150–300 µg/ml and the dose of tacrolimus was adjusted to achieve a trough serum concentration of 5–15 ng/ml. Cyclosporine and tacrolimus were tapered beginning at days + 100 depending on GVHD status. Mycophenolate mofetil was taken orally from day 0 at a dose of 600 mg/m2 per day in divided doses and was tapered to discontinuation between days + 30 and + 60. MTX was administered intravenously at a dose of 15 mg/m2 on days + 1 and 10 mg/m2 on days + 3, + 6 and + 11. Cytomegalovirus (CMV) DNA in blood samples was monitored weekly by real-time PCR. Once the CMV copies were more than 400/ml in two independent tests, ganciclovir or foscarnet combined with γ-globulin was given. Some patients received posttransplant maintenance therapy to prevent relapse, including tyrosine kinase receptor inhibitors for patients diagnosed with CML, Philadelphia chromosome-positive ALL and AML with KIT mutation, demethylating agents for patients diagnosed with AML without target drugs available, and chidamide for patients diagnosed with T-ALL.
Definition of disease stage
Disease stage was defined according to our and others’ published literature [5, 20, 21]. Early-stage disease was defined as CML in the first chronic phase, de novo acute leukemia in CR1, MDS-RA, MDS-RARS, CLL and lymphoma with chemotherapy-sensitive disease or the most recent relapse-free interval greater than 6 months. Late-stage disease was defined as CML in the accelerated phase or in the second chronic phase, secondary/therapy-related acute leukemia in CR1, acute leukemia in the second or third remission, MDS-EB, lymphoma with disease that was not regarded as chemotherapy-sensitive or the most recent relapse interval was 6 months or less. Active disease was defined as CML in the blast phase, acute leukemia without remission, and lymphoma with over 20% tumor cells in the bone marrow.
Statistics
Patient characteristics are expressed as the median and range for continuous variables, and the difference between groups was tested by the Mann–Whitney method. Categorical variables are expressed as frequencies. The differences between groups were tested by the chi square or Fisher’s exact test, and multivariate analysis was conducted by a logistical regression model.
Overall survival (OS) was measured from transplantation to death from any cause. Recurrence-free survival (RFS) was defined as survival without disease recurrence. GVHD-free relapse-free survival (GRFS) was defined as survival without grade III-IV acute GVHD (aGVHD) or chronic GVHD (cGVHD) requiring systematic treatment or disease recurrence. OS, RFS and GRFS were estimated by the Kaplan–Meier method. Univariate comparisons were performed using the log-rank test, and the Cox proportional hazards regression model was used for multivariate analysis.
Neutrophil engraftment was defined as an absolute neutrophil count of at least 500/µl for 3 consecutive days after transplantation. Neutrophil engraftment at days +28, cumulative incidence of relapse (CIR), nonrelapse mortality (NRM), aGVHD and cGVHD were estimated by a competing risk model. Death was regarded as a competing event for neutrophil engraftment, aGVHD and cGVHD. In addition, DLI and secondary transplantation were also considered competing events for aGVHD and cGVHD. NRM and relapse were competing events for each other. Univariate significance was estimated by Gray’s K-sample test, and multivariate analysis was conducted by competing risk regression.
All factors with a P value < 0.1 by univariate analysis were included in the multivariate analysis. In addition, ATG preparation and HLA match were entered into the multivariate analysis regardless of the P value in the univariate analysis. Mann–Whitney, chi square test, Fisher’s exact test, logistical regression model, Kaplan–Meier and Cox regression models were performed with SPSS 23.0. Cumulative incidences were computed with R functions from the package cmprsk (R version × 64 4.1.2, package cmprsk version 2.2–11).
Results
Patient characteristics
A total of 186 patients were included in this study. One hundred and seven patients received ATG-F and seventy-nine patients received ATG-G. There were no significant differences between the patients who received ATG-F and the patients who received ATG-G with respect to patient sex, age, disease stage, donor-recipient sex, conditioning regimen, HLA match, infused mononuclear cell number, infused CD34 + cell number or GVHD prophylaxis (Table 1). The patient diagnosis was significantly different between the ATG-F group and the ATG-G group, with acute leukemia with ambiguous lineage (ALAL), acute lymphoblastic leukemia (ALL), acute myeloid leukemia (AML), chronic lymphocytic leukemia (CLL), chronic myelogenous leukemia (CML), marrow dysplastic syndrome (MDS) and non-Hodgkin’s lymphoma (NHL) proportions of 3.7% vs. 5.1%, 27.1% vs. 20.3%, 39.3% vs. 54.4%, 0.9% vs. 0%, 21.5% vs. 7.6%, 6.5% vs. 7.6%, and 0.9% vs. 5.1%, respectively (P = 0.032).
Engraftment
The cumulative incidence of neutrophil engraftment at days +28 in the ATG-F group and the ATG-G group was similar (96.3% vs. 94.9%, P = 0.571, Fig. 1A). Multivariate analysis showed that the type of ATG preparation had no impact on neutrophil engraftment (P = 0.61, Table 3). Receiving CCNU/MECCNU + Ara-c + BU + CY as a conditioning regimen was an independent risk factor for neutrophil engraftment (HR = 0.607 and P = 0.005, Table 3).
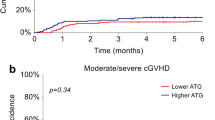
A Neutrophil engraftment at days +28, B cumulative incidence of relapse, C nonrelapse mortality, D cumulative incidence of grade II-IV acute GVHD, E cumulative incidence of chronic GVHD and F cumulative incidence of extensive chronic GVHD for patients receiving ATG-Fresenius and patients receiving ATG-Genzyme
Relapse and nonrelapse mortality
CIR in the ATG-F group and the ATG-G group was not significantly different (33.5% vs. 19.4%, P = 0.153, Fig. 1B). Factors affecting the CIR included pretransplant disease stage, GVHD prophylaxis, cGVHD and maintenance therapy (P = 0.043, P = 0.0495, P = 0.008, P = 0.049, respectively, Table 2). Multivariate analysis showed that the type of ATG preparation had no effect on CIR (P = 0.092, Table 3). Pretransplant active disease and the use of FK506 + MMF + MTX as GVHD prophylaxis were independent risk factors for relapse (HR = 3.371 and P = 0.028 and HR = 2.93 and P = 0.024, respectively, Table 3), while extensive cGVHD was a preventative factor for relapse (HR = 0.23, P = 0.017, Table 3).
NRM in the ATG-F group and the ATG-G group was not significantly different (10.4% vs. 15.0%, P = 0.402, Fig. 1C), but it was significantly affected by acute GVHD (P = 0.007, Table 2). Grade III-IV aGVHD was the only risk factor for NRM in the multivariate analysis (HR = 5.602, P < 0.001, Table 3).
GVHD
Univariate analysis showed that the type of ATG preparation had no effect on the cumulative incidence of grade II-IV aGVHD (8.4% vs. 6.3%, P = 0.583, Fig. 1D). HLA mismatch was the only factor affecting the incidence of grade II-IV aGVHD (P = 0.041, Table 2). Multivariate analysis showed that the type of ATG preparation had no effect on grade II-IV aGVHD (P = 0.47, Table 3). HLA mismatch was a risk factor for grade II-IV aGVHD (HR = 3.069, P = 0.041, Table 3).
Univariate analysis showed no impact of the type of ATG preparation on the cumulative incidence of cGVHD (43.9% vs. 28.8%, P = 0.279, Fig. 1E). Multivariate analysis showed that neither of the ATG preparations was a risk factor for cGVHD (P = 0.29, Table 3).
There was a trend toward a higher incidence of extensive cGVHD in patients receiving ATG-F (30.5% vs. 17.6%, P = 0.092, Fig. 1F), and a higher incidence of extensive cGVHD in patients with cytomegaloviremia (P = 0.03, Table 2). Multivariate analysis showed that ATG-G was a favorable factor and cytomegaloviremia was a risk factor for extensive cGVHD (HR = 0.41 and P = 0.01, and HR = 2.58 and P = 0.003, respectively, Table 3).
Cytomegaloviremia
The incidence of cytomegaloviremia was significantly higher in the ATG-G group (64.6% vs. 29.9%, P < 0.001) and in patients receiving posttransplant maintenance therapy (P = 0.005, Table 2). Multivariate analysis showed that ATG-G and grade III-IV aGVHD were independent risk factors for cytomegaloviremia (HR = 4.244 and P < 0.001, and HR = 6.695 and P = 0.034, respectively, Table 3).
Survival
There was no significant difference in OS between patients receiving ATG-F and patients receiving ATG (75% vs. 80.9%, P = 0.645, Fig. 2A). Factors affecting OS included pretransplant disease stage, aGVHD and cGVHD (P = 0.001, P = 0.038, and P = 0.036, respectively, Table 4). Multivariate analysis showed that the type of ATG preparation had no impact on OS (P = 0.795, Table 4). Pretransplant active disease and grade III-IV aGVHD were risk factors for OS (HR = 3.462 and P = 0.01, and HR = 4.548 and P = 0.016, respectively), and extensive cGVHD was a favorable factor for OS (HR = 0.279, P = 0.042).
RFS did not significantly differ between patients receiving ATG-F and patients receiving ATG (56.2% vs. 65.5%, P = 0.564, Fig. 2B). Pretransplant disease stage, aGVHD, cGVHD and maintenance therapy were factors affecting RFS (P = 0.001, P = 0.028, P = 0.001, and P = 0.007, respectively, Table 4). Multivariate analysis showed that the type of ATG preparation did not affect RFS (P = 0.945, Table 4). Factors affecting RFS included pretransplant active disease (HR = 2.607, P = 0.025), grade III-IV aGVHD (HR = 3.772, P = 0.009), limited cGVHD (HR = 0.287, P = 0.042), extensive cGVHD (HR = 0.251, P = 0.004) and maintenance therapy (HR = 0.296, P = 0.046).
There was no significant difference in GRFS between patients receiving ATG-F and patients receiving ATG (33.5% vs. 52.8%, P = 0.109, Fig. 2C). Multivariate analysis showed that the type of ATG preparation was not an independent influential factor for GRFS (P = 0.082, Table 4).
Discussion
In this article, we retrospectively analyzed 186 patients with hematological malignancies who underwent unrelated donor transplantation and compared the outcomes of 107 patients who received ATG-F 20 mg/kg in their transplant procedure with those of 79 patients who received ATG-G 10 mg/kg. There was no significant difference in the rates of engraftment, relapse, or NRM, the cumulative incidence of grade II-IV aGVHD or cGVHD, OS, RFS or GRFS between patients receiving ATG-F and patients receiving ATG-G. However, compared with ATG-F, ATG-G was associated with a lower risk of extensive cGVHD and a higher incidence of CMV reactivation. The more potent immunosuppressive effect of ATG-G at a dose of 10 mg/kg compared to ATG-F at a dose of 20 mg/kg may account for this finding. Two studies demonstrated that ATG-G 10 mg/kg was correlated with delayed T-cell reconstitution in comparison with ATG-F 25 mg/kg and 45–60 mg/kg [22, 23]. The broad antibody spectrum of ATG-G may also be related to its association with less extensive cGVHD and a higher rate of cytomegaloviremia. ATG-G is a polyclonal antibody that also targets molecules on B cells, such as CD19 and CD20 [24].
It is well known that ATG is associated with CMV reactivation [25], which can lead to serious complications after transplantation. Letermovir can reduce the morbidity and mortality associated with CMV reactivation, but it is too expensive for many patients in developing areas to afford. The differences in cytomegaloviremia incidence between patients receiving different ATG products suggest that the prevention of posttransplant CMV reactivation could be adjusted according to the ATG product.
The difference in the risk of extensive cGVHD between patients who received ATG-F and those who received ATG-G suggest that the selection of ATG preparation should be based on the incidence of extensive cGVHD of each center. Adjustment of the posttransplant management strategy based on ATG preparation, for example, delaying the discontinuation of calcineurin inhibitors for patients who receive ATG-Fresenius, is recommended.
In previous studies comparing ATG-F and ATG-G in unrelated transplantation at fixed doses, Huang et al. used the same dose of ATG as we did [16, 17]. However, in contrast to our findings, they observed a lower cGVHD incidence in patients treated with ATG-F. This discrepancy may be caused by the inadequate number of patients included and the absence of multivariate analysis in their study.
Although studies have shown no difference in T-cell reconstitution and similar GVHD incidence between patients who receiving ATG-F 60 mg/kg and those who receive 45 mg/kg [22], more studies support that lowering the dose of ATG-F improves survival. A retrospective study by Ayuk et al. compared ATG-F 30 mg/kg and 60 mg/kg in cases of unrelated matched transplantation. The results showed that ATG dose had no effect on the rate of GVHD or relapse, but the lower dose was associated with a decreased rate of fatal infection and TRM and increased DFS [26]. ATG-F 35 mg/kg and 60 mg/kg for elderly patients receiving unrelated donor transplantation was compared with no ATG by Binkert et al. TRM and survival in the lower dose group were superior to those in the no ATG group, while the higher dose showed no advantage [27]. A multicenter phase 3 randomized clinical trial by Locatelli et al. showed that there was no difference in the rate of grade II-IV acute GVHD, NRM or the relapse rate between children who received unrelated donor transplantation with ATG-F 15 mg/kg and those who received ATG-F 30 mg/kg. However, the lower dose was related to an increased 5-year OS and EFS [28]. Therefore, reducing the dose of ATG-F may improve survival without increasing the risk of GVHD. In this article, despite the higher risk of extensive cGVHD in patients receiving ATG-F 20 mg/kg, their aGVHD incidence, overall cGVHD incidence and survival were similar to those of patients receiving ATG-G 10 mg/kg.
Analysis of posttransplant lymphocyte subsets clearly showed that higher doses of ATG-G, but not ATG-F, led to delayed immune reconstitution [22, 29, 30]. Therefore, theoretically, higher doses of ATG-G may increase the risk of infection and relapse, but studies have shown an inconsistent effect of higher doses of ATG-G on survival. A randomized controlled clinical study by Wang et al. showed that with a lower incidence of grade III-IV aGVHD, the ATG-G 10 mg/kg group had a similar 1-year DFS to the ATG-G 6 mg/kg group in haploidentical transplantation [31]. The long-term follow-up results of another prospective randomized clinical study showed that although ATG-G 10 mg/kg increased the risk of infection in haploidentical transplantation compared to ATG-G 6 mg/kg, the incidence of cGVHD was decreased, and GRFS was improved [32]. A retrospective study by Devillier et al. found that in reduced-intensity conditioning matched sibling donor transplantation, doses of ATG-G higher than 6 mg/kg led to an adverse impact on survival compared with doses less than 6 mg/kg because of an increased rate of relapse [33]. A randomized controlled clinical study found that 7.5 mg/kg ATG-G did not decrease the incidence of grade III-IV aGVHD. Furthermore, 15 mg/kg ATG-G reduced the incidences of grade III-IV aGVHD and extensive cGVHD but did not improve survival due to an increased incidence of fatal infection compared to no ATG [34]. The contradictory findings of the above study related to higher doses of ATG-G may have been caused by differences in the optimal dose of ATG determined for different donor sources and conditioning intensities. The similar relapse rate and NRM and decreased risk of extensive cGVHD in the ATG-G 10 mg/kg group compared with those in the ATG-F 20 mg/kg group in this study indicate that ATG-G at a dose of 10 mg/kg may be appropriate for unrelated hematopoietic stem cell transplantation.
This study has limitations as a retrospective study. First, it included patients with several diseases because no single disease group had enough patients for analysis, and the disease distribution was different between the ATG-F group and the ATG-G group, which may have influenced the analysis results since the risk stratification for different diseases was heterogeneous. Second, the NIH chronic cGVHD scoring system was not used in this study to distinguish the severity of cGVHD since the cGVHD score for some patients was not available. Finally, although the case number in this study is the largest of studies comparing ATG-F and ATG-G, it is still relatively small.
In conclusion, the results of this study suggest that ATG-G at a dose of 10 mg/kg is more effective in reducing extensive cGVHD than ATG-F at a dose of 20 mg/kg in unrelated hematopoietic stem cell transplantation but increases the risk of cytomegaloviremia. Selection of the ATG preparation according to the incidence of extensive cGVHD of each center and adjustment of the posttransplant management strategy according to the ATG preparation are recommended.
References
Bacigalupo A et al (2006) Thymoglobulin prevents chronic graft-versus-host disease, chronic lung dysfunction, and late transplant-related mortality: long-term follow-up of a randomized trial in patients undergoing unrelated donor transplantation. Biol Blood Marrow Transplant 12(5):560–565
Finke J et al (2009) Standard graft-versus-host disease prophylaxis with or without anti-T-cell globulin in haematopoietic cell transplantation from matched unrelated donors: a randomised, open-label, multicentre phase 3 trial. Lancet Oncol 10(9):855–864
Socié G et al (2011) Chronic graft-versus-host disease: long-term results from a randomized trial on graft-versus-host disease prophylaxis with or without anti-T-cell globulin ATG-Fresenius. Blood 117(23):6375–6382
Finke J et al (2017) Long-term outcomes after standard graft-versus-host disease prophylaxis with or without anti-human-T-lymphocyte immunoglobulin in haemopoietic cell transplantation from matched unrelated donors: final results of a randomised controlled trial. Lancet Haematol 4(6):e293–e301
Walker I et al (2016) Pretreatment with anti-thymocyte globulin versus no anti-thymocyte globulin in patients with haematological malignancies undergoing haemopoietic cell transplantation from unrelated donors: a randomised, controlled, open-label, phase 3, multicentre trial. Lancet Oncol 17(2):164–173
Kröger N et al (2016) Antilymphocyte globulin for prevention of chronic graft-versus-host disease. N Engl J Med 374(1):43–53
Kim HJ et al (2009) Successful prevention of acute graft-versus-host disease using low-dose antithymocyte globulin after mismatched, unrelated, hematopoietic stem cell transplantation for acute myelogenous leukemia. Biol Blood Marrow Transplant 15(6):704–717
Kawamura K et al (2017) Impact of the presence of HLA 1-locus mismatch and the use of low-dose antithymocyte globulin in unrelated bone marrow transplantation. Bone Marrow Transplant 52(10):1390–1398
Soiffer RJ et al (2017) Prospective, randomized, double-blind, phase iii clinical trial of anti-T-lymphocyte globulin to assess impact on chronic graft-versus-host disease-free survival in patients undergoing HLA-matched unrelated myeloablative hematopoietic cell transplantation. J Clin Oncol 35(36):4003–4011
Rubio MT et al (2016) The impact of HLA-matching on reduced intensity conditioning regimen unrelated donor allogeneic stem cell transplantation for acute myeloid leukemia in patients above 50 years-a report from the EBMT acute leukemia working party. J Hematol Oncol 9(1):65
Ayuk F et al (2009) Comparison of the cytotoxicity of 4 preparations of anti-T-cell globulins in various hematological malignancies. Anticancer Res 29(4):1355–1360
Penack O et al (2007) The type of ATG matters – natural killer cells are influenced differentially by Thymoglobulin Lymphoglobulin and ATG-Fresenius. Transpl Immunol 18(2):85–87
Préville X et al (2000) A quantitative flow cytometry assay for the preclinical testing and pharmacological monitoring of rabbit antilymphocyte globulins (rATG). J Immunol Methods 245(1–2):45–54
Remberger M et al (1999) Effect on cytokine release and graft-versus-host disease of different anti-T cell antibodies during conditioning for unrelated haematopoietic stem cell transplantation. Bone Marrow Transplant 24(8):823–830
Basara N et al (2005) Antithymocyte globulin for the prevention of graft-versus-host disease after unrelated hematopoietic stem cell transplantation for acute myeloid leukemia: results from the multicenter German cooperative study group. Bone Marrow Transplant 35(10):1011–1018
Huang W et al (2016) The efficacy and safety of rabbit anti-thymocyte globulin vs rabbit anti-T-lymphocyte globulin in peripheral blood stem cell transplantation from unrelated donors. Leuk Lymphoma 57(2):355–363
Huang W et al (2015) Outcomes of peripheral blood stem cell transplantation patients from HLA-mismatched unrelated donor with antithymocyte globulin (ATG)-Thymoglobulin versus ATG-Fresenius: a single-center study. Med Oncol 32(2):465
Polverelli N et al (2018) Comparative study on ATG-thymoglobulin versus ATG-fresenius for the graft-versus-host disease (GVHD) prophylaxis in allogeneic stem cell transplantation from matched unrelated donor: a single-centre experience over the contemporary years. Leuk Lymphoma 59(11):2700–2705
Zhang XH et al (2021) The consensus from The Chinese Society of Hematology on indications, conditioning regimens and donor selection for allogeneic hematopoietic stem cell transplantation: 2021 update. J Hematol Oncol 14(1):145
Walker I et al (2020) Addition of anti-thymocyte globulin to standard graft-versus-host disease prophylaxis versus standard treatment alone in patients with haematological malignancies undergoing transplantation from unrelated donors: final analysis of a randomised, open-label, multicentre, phase 3 trial. Lancet Haematol 7(2):e100–e111
Chen XH et al (2009) Role of antithymocyte globulin and granulocyte-colony stimulating factor-mobilized bone marrow in allogeneic transplantation for patients with hematologic malignancies. Biol Blood Marrow Transplant 15(2):266–273
Oostenbrink LVE et al (2019) Differential elimination of anti-thymocyte globulin of Fresenius and Genzyme impacts T-cell reconstitution after hematopoietic stem cell transplantation. Front Immunol 10:315
Terasako K et al (2010) The effect of different ATG preparations on immune recovery after allogeneic hematopoietic stem cell transplantation for severe aplastic anemia. Hematology 15(3):165–169
Gaber AO et al (2010) Rabbit antithymocyte globulin (thymoglobulin): 25 years and new frontiers in solid organ transplantation and haematology. Drugs 70(6):691–732
Luo X-H et al (2021) CMV infection and CMV-specific immune reconstitution following haploidentical stem cell transplantation: an update. Front Immunol 12:732826
Ayuk F et al (2008) Comparison of two doses of antithymocyte globulin in patients undergoing matched unrelated donor allogeneic stem cell transplantation. Biol Blood Marrow Transplant 14(8):913–919
Binkert L et al (2015) Lower dose anti-thymocyte globulin for GvHD prophylaxis results in improved survival after allogeneic stem cell transplantation. Bone Marrow Transplant 50(10):1331–1336
Locatelli F et al (2017) Efficacy of two different doses of rabbit anti-T-lymphocyte globulin to prevent graft-versus-host disease in children with haematological malignancies transplanted from an unrelated donor: a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 18(8):1126–1136
Liu J et al (2015) Differential impact of two doses of antithymocyte globulin conditioning on lymphocyte recovery upon haploidentical hematopoietic stem cell transplantation. J Transl Med 13:391
Lindemans CA et al (2014) Impact of thymoglobulin prior to pediatric unrelated umbilical cord blood transplantation on immune reconstitution and clinical outcome. Blood 123(1):126–132
Wang Y et al (2014) Influence of two different doses of antithymocyte globulin in patients with standard-risk disease following haploidentical transplantation: a randomized trial. Bone Marrow Transplant 49(3):426–433
Chang YJ et al (2017) Optimal dose of rabbit thymoglobulin in conditioning regimens for unmanipulated, haploidentical, hematopoietic stem cell transplantation: Long-term outcomes of a prospective randomized trial. Cancer 123(15):2881–2892
Devillier R et al (2018) Impact of antithymocyte globulin doses in reduced intensity conditioning before allogeneic transplantation from matched sibling donor for patients with acute myeloid leukemia: a report from the acute leukemia working party of European group of Bone Marrow Transplantation. Bone Marrow Transplant 53(4):431–437
Bacigalupo A et al (2001) Antithymocyte globulin for graft-versus-host disease prophylaxis in transplants from unrelated donors: 2 randomized studies from Gruppo Italiano Trapianti Midollo Osseo (GITMO). Blood 98(10):2942–2947
Funding
This research was supported by National Natural Science Foundation of China (No. 82170161), Natural Science Foundation Project of Chongqing (cstc2020jcyj-msxmX0756), and the Research Project of Postgraduate Education and Teaching Reform (2022yjgA08).
Author information
Authors and Affiliations
Contributions
LeG and LW designed the study. LW, LDZ, YMF, TC, JL, SCG, HFL and YXL collected the data, LW, PYK, CZ and LeG analyzed and interpreted the data. LW and LeG wrote the manuscript. PYK, CZ, LiG, HY, YQL, LZ, and XZ reviewed and revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, L., Kong, P., Zhang, C. et al. Outcomes of patients with hematological malignancies who undergo unrelated donor hematopoietic stem cell transplantation with ATG-Fresenius versus ATG-Genzyme. Ann Hematol 102, 1569–1579 (2023). https://doi.org/10.1007/s00277-023-05220-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-023-05220-7