Abstract
Acquired thrombotic thrombocytopenic purpura (aTTP) is a rare disease with an acute and severe clinical presentation. The anti-von Willebrand factor caplacizumab was licensed for adults with aTTP based on prospective controlled trials. However, until now, there was no Brazilian experience with this new treatment modality. This retrospective, multicenter, single-arm, expanded access program (EAP) with caplacizumab, plasma exchange (PEX), and immunosuppression was conducted between 02/24/21 and 04/14/21, and enrolled 5 Brazilian patients with aTTP. EAP allowed access to caplacizumab in Brazil and real-world data was collected, at a time when the medication was not commercially available in Brazil. The median age was 31 years old, most patients were women (80%), and neurological manifestation was observed in 80% of cases. The median of laboratory tests was hemoglobin (Hb) of 11 g/dL, platelets (16.1 × 109/L), lactic dehydrogenase (LDH) of 1471 U/L, creatinine (0.7 mg/dL), ADAMTS13 activity lower than 0.71%, and PLASMIC score of 6. All patients received immunosuppression, PEX, and caplacizumab. Until clinical response was achieved, the median was 3 sessions of PEX and 3 days of treatment. The median time of caplacizumab use was 35 days, with platelet normalization in 2 days after starting the drug. The median total length of stay was 8 days. All patients achieved clinical response and clinical remission, with a good safety profile. There was rapid clinical response, few PEX sessions were necessary, and there were short hospital stay, absence of refractoriness, little exacerbation, no death, and resolution of signs and symptoms at diagnosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
aTTP is a rare and fatal disease if not diagnosed and treated early [1]. It is a type of thrombotic microangiopathy (TMA) characterized by microangiopathic hemolytic anemia (MAHA), severe thrombocytopenia, and end-organ damage resulting from thrombus formation in the microcirculation due to severe deficiency of the ADAMTS13 enzyme (disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13) [1]. The genesis of the enzyme deficiency in aTTP is autoimmune, with the presence, in most cases, of antibodies inhibiting the enzyme activity. Severe deficiency of the ADAMTS13 (< 10%) induces the accumulation of large von Willebrand factor (vWF) multimers with formation of platelet microthrombi in the systemic microcirculation [2].
The disease establishes acutely, with secondary clinical signs of anemia, thrombocytopenia, and damage to the microcirculation, with potential neurological, gastrointestinal, cardiac, and renal manifestations [3, 4]. The traditional treatment of aTTP since the 1990s is immunosuppression, especially with corticosteroid therapy, associated with PEX [5]. The lethality of aTTP without treatment is greater than 90% and it is drastically reduced (below 20%) when early treatment is initiated, and despite that, lethality in the acute episode is considerable [4, 6]. Furthermore, the number of refractory patients and especially the number of exacerbations is still very high in traditional treatment.
After the initial clinical suspicion, the scores that predict ADAMTS13 deficiency help in the diagnosis, allowing the early treatment when the patient has a pre-test score demonstrating intermediate or high probability for enzyme deficiency. The most used and validated scores are the PLASMIC and the French score [7].
Due to the need for a new therapeutic modality that addresses the formation of platelet microthrombus and aiming at better protection of the microcirculation [8], a new class of immunotherapy with monoclonal nanoantibody capable of blocking the interaction of platelet with vWF was developed. This new drug is caplacizumab, whose efficacy and safety have been proven by the TITAN (phase 2) [9] and HERCULES (phase 3) [10] studies. Caplacizumab associated with PEX and immunosuppression induces a faster platelet recovery and reduces refractoriness, the number of PEX sessions, hospitalization days, and, above all, exacerbations. As adverse events, there is a greater risk of mucocutaneous hemorrhagic manifestations; however, for most patients this is mild and easily manageable [10]. Rituximab can be introduced at the beginning of treatment or when there is refractoriness or relapse [11]. The EAP allowed the first Brazilian experience with this new treatment modality for aTTP.
The main objective of this study is to report the results of the 5 EAP cases where the caplacizumab was used associated with PEX and immunosuppression. Besides, it describes the inherent challenges in the use of a new drug, the possible adverse events presented, the benefits of aiding diagnosis, the monitoring of the enzymatic response, and, especially, the outcomes presented by each patient.
Materials and methods
The EAP was approved by National Health Surveillance Agency (ANVISA) on December 10, 2020, for compassionate use of caplacizumab with the proposal to include a total of 5 patients. In total, 5 centers participated in the program. Of these, 3 centers included the 5 patients in a period of 50 days (from 2/24/21 to 4/14/21): two patients from Hospital Erasto Gaertner – Curitiba/PR, one patient from Hospital Santa Lúcia – Brasília/DF, and two patients from HEMORIO – Rio de Janeiro/RJ.
Inclusion criteria covered suspected cases of aTTP in adult patients, initial episode or relapse, ideally with intermediate or high pre-test probability of severe ADAMTS13 deficiency.
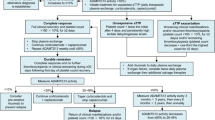
The protocol consisted, after signing the Free and Informed Consent Form (ICF), in collecting a plasma sample for analysis of ADAMTS13 activity and investigation of the inhibitor, and then immediate start of standard treatment with immunosuppression and PEX according to the protocol of each center. The requested medication was available within 24 h. Caplacizumab was administered with an intravenous (IV) loading dose of 10 mg before PEX followed by a subcutaneous (SC) dose of 10 mg after PEX. Subsequent doses (from D2 onwards) were 10 mg SC after PEX until clinical response, followed by a daily SC dose of 10 mg for 30 days after stopping PEX. At the end of this period, if there was evidence of unresolved immune disease, based on monitoring of ADAMTS13 activity, it was recommended to optimize the immunosuppression regimen and continue daily caplacizumab administration until signs of underlying immune disease were resolved.
Retrospective information in the medical records (physical and electronic) were evaluated. The variables of interest compiled for each patient were their demographic data, initial clinical and laboratory presentation, ADAMTS13 activity and inhibitor, PLASMIC score, concomitant immunosuppression, time to treatment response, number of days of PEX, time of caplacizumab use, response criteria, and adverse events.
The categorical variables (discrete or continuous) were described in a table. The data compiled from the 5 cases are represented as median for quantitative variables and as % for qualitative variables. The data regarding the clinical response of the 5 cases, in the first 30 days of treatment, were described in a graph. The project was approved on 09/29/21 by the Ethics and Research Committee and by the National Commission of Ethics in Research with a Certificate of Presentation of Ethical Appreciation registry 50,769,421.1.1001.5267.
Results
In a period of 50 days, 5 patients were included in the program, the majority being female (80%), with a median age of 31 years and all Caucasian. The median body mass index (BMI) was high (28) and as possible precipitating factors, the second patient had an upper respiratory infection 1 week before admission and the third patient had a relapse of TTP in the twentieth week of pregnancy, with a miscarriage requiring labor induction and curettage. Of these 5 cases, 2 were the first event and 3 were relapses.
At diagnosis, cutaneous and mucous hemorrhagic events were present in 100% of cases, and neurological manifestations in 80%, with a predominance of headache. Only 1 patient had confusion and reduced level of consciousness, with a Glasgow Coma Scale of 12. Anemic syndrome was present in 40% of cases and one patient had cardiac involvement, with positive troponin.
Anemia was mild in most cases, with a median hemoglobin of 11 g/dL. The median of platelets was 16.1 × 109/L and lactic dehydrogenase was 1471 U/L. Renal function and INR were normal and the median PLASMIC score was 6, with a high probability of enzyme deficiency. ADAMTS13 deficiency was confirmed in all cases, with a median of < 0.71% and a positive inhibitor test in four patients. In one case, the search for inhibitor was not possible due to sample submission failure.
Regarding the treatment instituted, all patients received immunosuppression, 100% with corticosteroid therapy, and 60% in association with rituximab (375 mg/m2 weekly for 4 weeks), but with a late onset, on the twentieth day of treatment. One patient received additional immunosuppression with cyclophosphamide (2 doses of 500 mg with an interval of 2 weeks) and vincristine (3 doses of 1 mg with an interval of 3 days) after completion of 4 doses of rituximab due to persistent severe enzyme deficiency, starting on the forty-second day of treatment. All patients underwent PEX, with a median of 5 daily sessions in total and 3 sessions until clinical response (platelet normalization). Of adverse event, there was only mild allergic reaction in 3 patients. All patients received caplacizumab, most starting on the same day as PEX, and in two cases starting 2 days after starting PEX. The median number of days of drug use was 35 days. Two patients required extended use of caplacizumab due to lack of enzymatic response. One patient did it for 58 days and the other for 63 days. Two patients had thrombocytosis during use, which resolved even before drug discontinuation, and there were no thromboembolic events. No other adverse events were reported. After hospital discharge, caplacizumab was maintained on an outpatient care, with daily SC administrations.
In the second case, caplacizumab was maintained on an outpatient care from Monday to Friday (outpatient administration on weekends was not possible) until 30 doses were completed. After 30 doses of caplacizumab, an enzyme activity of 53% was registered and the drug was discontinued, exactly on the same day of the positive diagnosis for COVID-19, which elapsed without any complications.
An unusual event that occurred in the third case is worth mentioning. In the patient who relapsed during pregnancy and miscarriage, on the third day after starting treatment, she presented fever and diffuse abdominal pain. Abdominal tomography revealed suggestive images of pyosalpingitis and pelvic abscesses. Caplacizumab and PEX were discontinued due to the need for a surgical approach. Four days after beginning of treatment, the patient already had clinical response criteria and was submitted to an exploratory laparotomy after a prophylactic dose of vWF (30 IU/kg). Exhaustive lavage of the cavity, drainage of abscesses, and curettage of placental debris were performed. There were no bleeding complications during surgery or postoperatively. As expected, on the second postoperative day, there was an exacerbation of TTP, indicating the need to restart PEX and caplacizumab, which achieved a rapid clinical response again.
Time to clinical response was 3 days after initiation of treatment and 2 days after initiation of caplacizumab. All patients achieved clinical response and sustained clinical remission; there were no death and refractoriness; and there was only one exacerbation in the context of interruption of PEX and caplacizumab due to the need for surgical intervention. The median length of hospital stay was 8 days, although the time to enzyme response (ADAMTS13 > 10%) was 33.5 days. All signs and symptoms presented at diagnosis were resolved, including complete recovery from the neurological condition of the fourth case, with discharge after only 8 days of hospitalization. As a probable sequel to the TTP event, one patient maintained systemic arterial hypertension after discharge and completion of treatment.
In the first case, it was not possible to assess the time for the enzymatic response because the patient refused to follow the monitoring from day 58, when the enzymatic activity was still low (3%). Despite that, the patient did not relapse after stopping the drug.
The fifth case took the longest time to reach the enzymatic response, which justified the prolonged use of caplacizumab (63 days). However, the patient achieved rapid clinical response (within 2 days) and had early hospital discharge, on the sixth day of hospitalization. The clinical and laboratory data and the responses to the treatments of the 5 cases are detailed in Tables 1 and 2. The clinical response of each case is represented in Fig. 1, considering the first 30 days of treatment.
Time (days) to clinical response after initiation of treatment. Legend: Treatment initiated on d0 consisted of at least plasmapheresis and immunosuppression. In cases 1, 4, and 5, caplacizumab was started on d0. In cases 2 and 3, it was started from d2, with a later clinical response. Clinical response is defined as platelet normalization. The median response after starting treatment was 3 days and after starting caplacizumab, 2 days
Discussion
The Brazilian experience with caplacizumab reproduced the main results of international registries and pivotal studies. The estimated incidence of aTTP varies from 2.9 to 13 cases per million per year, according to the English, French, and Oklahoma registries [2, 4, 12, 13]. Despite being a rare disease, in a short time (50 days) it was possible to include the five patients in the EAP with only 5 centers involved.
There is a predominance of females in the approximate ratio of 2 to 3:1, with involvement of individuals especially in the third and fourth decade of life [1, 2, 13]. In our experience, even greater predominance of females was registered (4:1) and even in younger patients, with a median of 31 years. All patients were Caucasians, coinciding with European records [2, 12]. We observed a predominance of obese patients, which corroborates obesity as a risk factor for aTTP [14], as well as a common comorbidity to develop after the acute episode; therefore, body mass index > 25 is common in the first episode and relapses.
The median hemoglobin ranges from 7.7 to 8.7 g/dL in the main registries [2, 12, 15] and this median was not observed in these cases, probably due to early diagnosis of some cases and previous transfusion of red blood cells before admission to the centers where the patients were treated. Thrombocytopenia is generally severe, with a median ranging from 15 to 17 × 109/L [12], and the median of 16.1 × 109/L in EAP cases corroborates this finding. Clinical manifestations secondary to anemia and to thrombocytopenia are very common and may be the only ones present at diagnosis [1]. Two of the five patients had anemic syndrome at diagnosis. The hemorrhagic manifestations, present in more than 50% of the patients in the registries [12, 15], were present in 100% of the EAP patients.
Symptoms secondary to ischemic events are present in up to 80% of patients at diagnosis and further increase the suspicion of aTTP in a patient with AHMA and severe thrombocytopenia [12]. Neurological complaints are more common, with progressive worsening over the hours and days when there is no effective treatment [2, 6, 12, 15, 16]. Four (80%) patients had neurological symptoms, especially headache (60%). Only one had a lowered level of consciousness, reflecting high-risk aTTP. Cardiac involvement is frequent, but subclinical in most cases [15]. It is more commonly observed through electrocardiographic findings and especially by elevation of troponin [12]. One patient had cardiac involvement due to elevation of troponin, also configuring high-risk aTTP. The English registry showed that GCS < 15 and elevated troponin are isolated risk factors and increase mortality by 9 and 6 times, respectively [6].
Gastrointestinal manifestations are also very common, such as nausea, vomiting, abdominal pain, and diarrhea [2, 15]; however, they were not reported by any patient. The kidneys are also a target in aTTP, but their involvement is not as common as seen in other forms of TMA [1, 3]. Renal impairment is usually mild, limited to stages 1 to 3, with no severe renal failure and no need for dialysis at diagnosis [1, 3]. The EAP data corroborate this information with a median creatinine of 0.7 mg/dL.
The PLASMIC score [7], widely used in clinical practice to assess the pre-test probability of severe ADAMTS13 deficiency, was effective in these 5 cases, with intermediate probability in 2 cases (5 points) and high probability in 3 cases (6–7 points), as we usually observe in the most typical cases of aTTP. Severe enzyme deficiency was confirmed in the 5 patients with the presence of inhibitor detected in 4 cases. In one case, the search for inhibitor was not possible due to sample submission failure; however, due to the clinical context and the prevalence of aTTP, the case was treated as acquired. The median of ADAMTS13 activity was very low (< 0.71%), which may reflect potentially more severe cases due to a longer delay in the response to immunosuppressive treatment.
All patients received the three pillars of treatment for aTTP. Five patients (100%) received corticosteroid therapy, 3 (60%) received rituximab, and 1 (20%) required additional immunosuppression with vincristine and cyclophosphamide due to lack of immune response with corticosteroids and rituximab. The use of the rituximab, recommended in initial association with corticosteroids specially to reduce exacerbations and relapses, is not a reality for all Brazilian cases, mainly in public medicine. Due to difficulty in accessing the medication, it is usually used in selected cases such as relapsed aTTP, high-risk aTTP, or late response to the traditional treatment. These three cases started rituximab late, with a median of 20 days of treatment, especially due to the absence of an immune response evidenced by the monitoring of enzyme activity.
The possibility of monitoring the enzymatic response is a fundamental new concept in the management of patients with aTTP. It allows early interruption of treatment with caplacizumab in rapid responders and guides the extension of treatment and intensification of immunosuppression for late responders. The median time to reach enzyme activity above 10% was 33.5 days, although this assessment is biased as it depends on how often ADAMTS13 is monitored. The delay in achieving the immune response may be explained by the severity of ADAMTS13 deficiency at diagnosis and by the late use of rituximab. In any case, this time was not very different from what some real-life studies reported. In the French study [8], the median time to reach enzyme activity above 20% was 28 days in the caplacizumab group (100% used first-line rituximab) and 48 days in the historical control (68% used rescue rituximab).
All patients were submitted to PEX, with a median of 5 sessions in total and 3 sessions until clinical response. Usually, with the traditional treatment, longer times for clinical response and the need for a greater number of PEX sessions are observed. In a short period, EAP patients were free from PEX sessions, with early catheter removal and without serious adverse events associated with the procedure.
All patients used caplacizumab, starting immediately in most cases. In cases 2 and 3, caplacizumab was started 2 days after initiation of PEX and the time to clinical response was longer, 6 and 4 days respectively. After starting caplacizumab, the median for clinical response was 2 days, corroborating the rapid response data from the pivotal studies [9, 10]. The median duration of caplacizumab use was 35 days. In two cases, the extension to 58 and 63 days was necessary due to delay in the immune response. The use of caplacizumab in these cases, without PEX for several days and still with severe enzyme deficiency, allowed sustained response and microcirculation protection and prevented exacerbations and the need for readmission. There were no serious adverse events, not even bleeding events as expected. Even the patient who required an urgent surgical approach, with the empirical prophylactic dose of vWF, had no bleeding complications during the surgery or postoperative period, even after the reintroduction of caplacizumab in the face of exacerbation.
All patients submitted to the three pillars of treatment achieved clinical response and clinical remission, with a good safety profile. There were no refractoriness, death, thromboembolic event, adverse events, or serious sequelae. All signs and symptoms presented at diagnosis were resolved and most required a short hospital stay (median of 8 days). The only exacerbation occurred due to the treatment interruption and surgical and inflammatory stress, and when PEX and caplacizumab were restarted, there was again a rapid response.
Conclusion
With the peculiarities of the use of a new drug in the reality of Brazilian public medicine, the experience with the use of caplacizumab in patients with aTTP reproduced well the results observed in the pivotal studies and in the main aTTP registries. There was rapid clinical response, few PEX sessions were necessary, and there were short hospital stay, absence of refractoriness, little exacerbation, no death, and resolution of signs and symptoms at diagnosis. In addition, there were no adverse events associated with the drug and the use of caplacizumab allowed time to immunosuppressive treatment response, keeping them protected especially in the period of low enzyme activity.
Abbreviations
- aTTP:
-
Acquired thrombotic thrombocytopenic purpura
- PEX:
-
Plasma exchange
- EAP:
-
Expanded access program
- Hb:
-
Hemoglobin
- LDH:
-
Lactic dehydrogenase
- TMA:
-
Thrombotic microangiopathy
- MAHA:
-
Microangiopathic hemolytic anemia
- ADAMTS13:
-
Disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13
- vWF:
-
Von Willebrand factor
- ANVISA:
-
National Health Surveillance Agency
- ICF:
-
Informed consent form
- IV:
-
Intravenous
- SC:
-
Subcutaneous
- GCS:
-
Glasgow Coma Scale
References
Chiasakul T, Cuker A (2018) Clinical and laboratory diagnosis of TTP: an integrated approach. Hematology Am Soc Hematol Educ Program 2018(1):530–538
Mariotte E, Azoulay E, Galicier L et al (2016) Epidemiology and pathophysiology of adulthood-onset thrombotic microangiopathy with severe ADAMTS13 deficiency (thrombotic thrombocytopenic purpura): a cross-sectional analysis of the French national registry for thrombotic microangiopathy. Lancet Haematol 3(5):e237–e245
George JN, Nester CM (2014) Syndromes of thrombotic microangiopathy. N Engl J Med 371(7):654–666
Hovinga JAK, Coppo P, Lämmle B, Moake JL, Miyata T, Vanhoorelbeke K (2017) Thrombotic thrombocytopenic purpura. Nat Rev Dis Primers 3:17020
Rock GA, Shumak KH, Buskard NA et al (1991) Comparison of plasma exchange with plasma infusion in the treatment of thrombotic thrombocytopenic purpura Canadian Apheresis Study Group. N Engl J Med 325(6):393–7
Alwan F, Vendramin C, Vanhoorelbeke K et al (2017) Presenting ADAMTS13 antibody and antigen levels predict prognosis in immune-mediated thrombotic thrombocytopenic purpura. Blood 130(4):466–471
Bendapudi PK, Upadhyay V, Sun L, Marques MB, Makar RS (2017) Clinical scoring systems in thrombotic microangiopathies. Semin Thromb Hemost 43(5):540–548
Coppo P, Bubenheim M, Azoulay E et al (2021) A regimen with caplacizumab, immunosuppression, and plasma exchange prevents unfavorable outcomes in immune-mediated TTP. Blood 137(6):733–742
Peyvandi F, Scully M, Hovinga JAK et al (2016) Caplacizumab for acquired thrombotic thrombocytopenic purpura. N Engl J Med 374(6):511–522
Scully M, Cataland SR, Peyvandi F et al (2019) Caplacizumab treatment for acquired thrombotic thrombocytopenic purpura. N Engl J Med 380(4):335–346
Coppo P (2017) Treatment of autoimmune thrombotic thrombocytopenic purpura in the more severe forms. Transfus Apher Sci 56(1):52–56
Scully M, Yarranton H, Liesner R et al (2008) Regional UK TTP registry: correlation with laboratory ADAMTS 13 analysis and clinical features. Br J Haematol 142(5):819–826
Reese JA, Muthurajah DS, Hovinga JAK, Vesely SK, Terrell DR, George JN (2013) Children and adults with thrombotic thrombocytopenic purpura associated with severe, acquired Adamts13 deficiency: comparison of incidence, demographic and clinical features. Pediatr Blood Cancer 60:1676–1682
Joly BS, Coppo P, Veyradier A (2019) An update on pathogenesis and diagnosis of thrombotic thrombocytopenic purpura. Expert Rev Hematol 12(6):383–395
George JN (2010) How I treat patients with thrombotic thrombocytopenic purpura: 2010. Blood 116(20):4060–4069
George JN (2018) The remarkable diversity of thrombotic thrombocytopenic purpura: a perspective. Blood Adv 2(12):1510–1516
Author information
Authors and Affiliations
Contributions
Tiago Boechat: responsible for providing information regarding the cases treated at his center, compiled all the data, performed the statistical analyses, wrote the complete article, formulated the tables, and revised the article.
João Samuel: responsible for providing information regarding the cases treated at their center and acted in the review of the article.
Eduardo Flávio: responsible for providing information regarding the cases treated at their center and acted in the review of the article.
Michaela Larissa: responsible for providing information regarding the cases treated at their center and acted in the review of the article.
Corresponding author
Ethics declarations
Competing interests
Tiago de Oliveira Boechat: participates as a member of the advisory board and as a speaker of Sanofi Company and received fee as compensation.
João Samuel de Holanda Farias: participates as a speaker of Sanofi Company and received fee as compensation.
Eduardo Flávio Oliveira Ribeiro: participates as a speaker of Sanofi Company and received fee as compensation.
Michaela Larissa Lobo de Andrade: nothing to declare.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
de Oliveira Boechat, T., de Holanda Farias, J.S., Ribeiro, E.F.O. et al. Brazilian experience with caplacizumab in acquired thrombotic thrombocytopenic purpura: outcomes of the expanded access program. Ann Hematol 102, 1581–1588 (2023). https://doi.org/10.1007/s00277-023-05211-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-023-05211-8