Abstract
Iron deficiency anemia (IDA) is a common health problem in developing countries. Many studies have shown that low-dose oral iron could have similar efficacy and less gastrointestinal effects in iron deficiency without anemia. This prospective open-labeled randomized controlled study was designed to compare the response of 200 mg ferrous fumarate thrice-weekly (TIW) as not inferior to the thrice-daily (TID) regimen and to assess the incidence of adverse events (AEs) between two regimens in treating adult patients with IDA. The primary endpoint was either an increase in Hb ≥ 3 g/dL, having Hb of 12 g/dL in females or 13 g/dL in males at the 12th week of treatment. Secondary outcomes included adverse events (AEs), red blood cell indices, iron profiles, and patient compliance. Sixty-four patients were randomized: 32 in the TIW arm and the other 32 in the TID arm. The response rates were not different between two arms either with intention to treat analysis (72.0%, 95%CI 56.6–88.5 vs. 71.9%, 95%CI 53.3–86.3, p = 0.777); or per-protocol analysis (88.9%, 95%CI 70.8–97.6 vs. 88.5%, 95%CI 69.8–97.6, p = 1.0), respectively. The trial demonstrated non-inferiority at a margin of 23%. Although the iron profile response of the TID arm was earlier than the TIW arm, almost all patients recovered from anemic symptoms at week 4, and hematologic responses were not different at week 12. There were more gastrointestinal AEs in the TID arm. In conclusion, this study showed that the TIW was non-inferior to the TID iron treatment of IDA patients but less AEs and costs.
Similar content being viewed by others
Introduction
Anemia is a significant cause of health loss, and the burden is high. In developing countries, iron deficiency anemia remains the major cause of anemia and morbidity worldwide [1]. The World Health Organization defines anemia as a hemoglobin (Hb) concentration below 13 g/dL in men over 15 years of age, below 12 g/dL in non-pregnant women over 15 years of age, and below 11 g/dL in second- and third-trimester pregnant women [2]. The diagnostic criteria for iron deficiency anemia vary between published studies [3]. Serum ferritin cut point for diagnosis of IDA ranged from 15 to 30 ng/mL, while 30 ng/mL is high sensitivity and specificity [4,5,6]. Because blood is iron-rich, IDA in adult patients can mostly result from chronic blood loss, and this is a common mechanism underlying the development of iron deficiency, for example, as a consequence of menstrual or significant gastrointestinal blood loss, as well as hookworm infestation [7]. Other contributory factors are malnutrition and medications, such as aspirin. Multiple medications such as antacids, histamine-2 receptor antagonists (H2RAs), proton pump inhibitors (PPIs), non-steroidal anti-inflammatory drugs (NSAIDs), aspirin, zinc, and manganese can contribute to the malabsorption of iron and/or increased risk of bleeding. Evidently, the drug interaction is no clinical significance [8].
The treatment of IDA aims to (i) restore normal circulating Hb level, (ii) replenish body iron store, (iii) improve quality of life, and (iv) improve physiological function. Successful iron replacement therapy (IRT) should achieve all these outcomes [7]. Traditionally, oral iron salts are taken in a split dose, two or three times a day. More recent data suggest that lower doses and infrequent administration may be as effective as the traditional regimen while probably associated with lower rates of adverse effects. In addition, it may be inconvenient for some people to find three periods during the day to take iron on an empty stomach [7, 9,10,11,12]. There is no strong study evaluating the effectiveness of lower dose regimens in patients with iron deficiency anemia. This study aimed to investigate whether thrice-weekly compared to a thrice-daily dose of oral 200 mg ferrous fumarate tablet for 12 weeks is inferior in terms of efficacy and safety for the treatment of IDA.
Methods
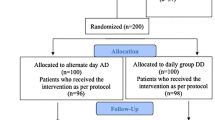
The prospective open-labeled randomized controlled study was conducted at Songklanagarind university hospital between February 1, 2020, and January 31, 2022. We recruited patients aged > 18 years and proven IDA with criteria of Hb < 12 g/dL in females or < 13 g/dL in males and serum ferritin level of < 30 ng/mL. They were excluded if having (i) a history of iron allergy, (ii) pregnancy or breastfeeding, (iii) a known history of inflammatory bowel disease, celiac disease, inherited bleeding disorder, solid cancer, hematologic cancer, or thalassemic disease, (iv) renal impairment with glomerular filtration rate less than 30 ml/min/1.73 m2, (v) hepatic impairment with a Child-Pugh score > 7, (vi) active bleeding as hemoglobin decrease > 2 g/dL, and (vii) received multivitamin or iron supplement > 35 mg of elemental iron per day within 2 weeks before randomization. They were considered to withdraw if they had ferrous fumarate intolerance, active bleeding, major surgery, received blood transfusion of more than 2 units, and loss to follow-up for longer than 2 weeks. One study author assessed the patients for study eligibility (LA). Another author (JS) was involved in generating the random allocation sequence, patient randomization, study drug administration, and ensuring compliance. Randomization was done with a 1:1 allocation in blocks of four to receive an oral 200 mg ferrous fumarate tablet either one-time thrice-weekly (TIW arm) on Monday, Wednesday, and Friday or one-time thrice-daily (TID arm) for 12 weeks of treatment. The protocol was approved by the institutional ethics committee (Ref no. BlB9-D4Zt-Oe66-6DGS, REC.62-404-14-1) and was registered at www.clinicaltrials.gov as NCT04130828.
The primary endpoint was defined as either an increase in Hb ≥ 3 g/dL or having Hb > 12 g/dL in females or > 13 g/dL in males after 12 weeks of treatment [13]. Secondary endpoints included adverse events (AEs), changes in red cell indices and iron profiles, and compliance. The patients were reassessed, and blood samples were taken at baseline, at 4 and 12 weeks. The red cell indices and iron profiles were run on XN-3000® (Sysmex Corporation) and Cobas 8000 c502® (Roche diagnostics), respectively. A structured interview was used to assess the symptoms of anemia with the particular list: fatigue, dyspnea on exertion, dizziness, sore tongue, taste disturbance, and pica at baseline, 4 and 12 weeks. All AEs subjectively reported by patients were recorded using the Common Terminology Criteria for Adverse Events version 4.0 in their logs daily and structured interviews on follow-up visits at weeks 4 and 12. Compliance was ensured by pill count, structured interviews, and hematological response on follow-up visits at weeks 4 and 12.
Sample size and statistical analysis
The sample size was estimated for the non-inferiority trial for the binary data using the therapeutic response rate of 92% [14], non-inferior margin (δ) using 25% of different response rate in the active comparator [15], 1:1 ratio, type I error of 0.05, and a power of 0.90. The result was calculated to be 24 in each arm [16, 17]. Assuming a drop-out rate of 20%, the accrual goal was 29 per arm. We compared the difference of variables with the chi-square test and Mann-Whitney test for categorical and continuous variables, respectively, and compared within-group effects by a linear mixed model analysis. The significance level was defined as P < 0.05, and all analysis was performed using IBM SPSS v22.
Results
Patients
Sixty-six patients were screened for eligibility, out of which a total of 64 were randomized (32 each in the TIW and TID arm; Fig. 1). Two became ineligible because of myelodysplastic syndromes. The baseline characteristics are shown in Table 1. The mean age was 49 years, and this was the same in both arms. Similarly, the patients in both treatment arms were predominantly females. The baseline characteristics, including age, gender, BMI, comorbidity, possible causes of IDA, concurrent medications used that may interfere with the results, anemic symptoms, hematological parameters, and iron profile were generally balanced between the two randomized treatments. Eight were lost to follow up and three withdrew from the study because they received blood transfusion > 2 units (2 patients) and oral iron treatment from another provider (1 patient). At the end of the 12-week duration of the study, the remaining number of patients in TIW and TID arms were 27 and 26, respectively (Table 2).
Primary endpoint
Although the mean Hb level in the TID arm was significantly higher than the TIW arm at week 4 (9.9 vs. 10.8 g/dL, respectively, p = 0.040), the mean Hb levels were not substantially different in either arm (11.9 vs. 12.4 g/dL, p = 0.188) at week 12 (Table 2). The primary endpoint in terms of an increase in Hb ≥ 3 g/dL or having Hb of 12 g/dL in females or 13 g/dL in males at 12th week of treatment was not significantly different between the two arms as revealed by both intentions to treat (ITT) analysis (72.0%, 95% CI 56.6–88.5 vs.71.9% 95% CI 53.3–86.3, respectively, p = 0.777) and per-protocol analysis (88.5%, 95%CI 69.8–97.6 vs. 88.9%, 95% CI 70.8–97.6, respectively, p = 1.000) (Table 3). The difference between the sample proportions was 0.1% (95%CI–21.9 to 22.1) with ITT analysis and 0.4% (95%CI–16.7 to 17.5) with per-protocol analysis. Since the 95% CIs did not contain the non-inferiority margin (δ) of 25%. The findings obtained by both protocols; the results indicated that the primary outcome (mean Hb levels) in the TIW arm was non-inferior relative to the TID arm.
With subgroup analysis for patients whose initial Hb level was less than 8 g/dL, the response rates were not significantly different between the two arms at week 4, i.e., 92.3% (95% CI 64.0–99.8) for the TIW arm (n = 13) vs. 92.9% (95% CI 66.1–99.8) for TID arm (n = 14), p = 1.000; and the same was true at week 12, 100% (n = 11) vs. 100% (n = 10), respectively, p = 1.000.
Secondary endpoints
Hematological parameters and iron profile within a group at weeks 4 and 12 are shown in Table 2 and between groups at weeks 4 and 12 are also shown in Table 4. Although the mean Hb levels in the TID arm were considerably higher than that of those in the TIW arm at week 4, the level was not clinically significant at the difference of 0.9 g/dL. Almost all recovered from anemic symptoms despite lesser Hb levels in the TIW arm (Table 5). Everyone was well and free of symptoms at week 12. We found that the mean RDWs were not different between both groups at weeks 4 and 12. While the mean MCVs were not different at week 4 in both arms (74.8 vs. 71.3 fL, p = 0.099), at week 12, it was significantly more in the TID arm than those in the TIW arm (80.6 vs. 74.5 fL, p = 0.012).
Regarding the iron profile of the treated patients, transferrin saturation was not different between the two arms at weeks 4 and 12. Serum ferritin levels in the TID arm were significantly greater than in the TIW arm at week 4 and 12. The proportion of patients who had serum ferritin > 30 ng/mL was lower in the TIW arm than in the TID arm at week 4 (44.8% vs. 73.3%, p = 0.035) but was not different at week 12 (64.3% vs. 73.1%, p = 0.430).
With regards to safety issues associated with the treatment, Table 6 summarizes the common AEs of oral ferrous fumarate in both arms. No severe or life-threatening AEs were reported in either arm. None of the patients discontinued the drug due to AEs. There were predominant gastrointestinal AEs, including nausea, epigastric discomfort, and metallic taste. Although the number of patients who got AEs was not statistically different between the two arms, total episodes of nausea and epigastric discomfort were much more in the TID arm.
Overall compliance was very high in both arms and was not different: 96.2% in the TIW arm vs. 93.1% in the TID arm (p = 0.200). Compliance at week 4 was 97.2% vs. 94.4% (p = 0.114); and at week 12, 95.4% vs. 92.0% (p = 0.154) in TIW and TID arms, respectively.
Discussion
Traditionally, oral iron salts for the treatment of IDA are taken in a divided dose of two or three times a day. Recent data suggest that a lower dose along with less frequent administration may be just as effective while probably associated with lower rates of adverse effects [7, 11, 12]. Most studies were conducted to prevent iron-deficient status in such females in reproductive life, pregnant women, and mothers during the breastfeeding period [18,19,20,21,22,23,24,25,26,27,28]. This study was designed to determine the response of treatment for IDA with oral ferrous fumarate administered thrice weekly (TIW), as not inferior to the thrice daily (TID) regimen, and compared the incidence of the adverse event between the two regimens. Our endpoint assessed the response at the end of the 12th week of treatment. Although the mean Hb level was slightly higher in the TID arm than the TIW arm (9.9 vs. 10.8 g/dL, respectively, p = 0.040) at week 4, there was no significant difference in clinical improvement. Almost all recovered from anemic symptoms. The therapeutic response rate at the end was not different between the two arms after 12 weeks of therapy with both intention-to-treat analysis (72.0% vs. 71.9%, respectively, p = 0.777) and per-protocol analysis (88.5%, vs. 88.9%, respectively, p = 1.000). The difference in therapeutic response rate between the two arms was 0.1%, 95%CI (−21.9 to 22.1) with ITT analysis and was 0.4%, 95%CI (−16.7 to 17.5) with per-protocol analysis. Since the 95% CIs did not contain the non-inferiority margin of 23%, these findings demonstrated positive results of the non-inferiority. The response rate was less than the previous study by Chaudhari DR et al. [14], in which the response proportion of ferrous sulfate 200 mg thrice daily after 12 weeks of treatment in adult IDA was 92.0%. Nevertheless, the response proportions in both arms were a little different from the study of Chaudhari DR et al. with per-protocol analysis. This was in accordance with the previous studies by Kaundal R et al. [29] in which the proportion of response classified by increased Hb level at ≥ 2 g/dL at week 3 and week 6 in the twice-daily arm was more than alternate-day arm. The response in the TIW arm at the 4th week was less than the previous systematic review by Okam MM et al. [30], in which the response proportion classified by Hb level at > 3 g/dL was 6.9%. Nevertheless, the response proportion in the 12th week of this study was more than their study (66.7%).
Concerning patients with severe anemia, having initial Hb < 8 g/dL, the response rates were not different in the TIW arm compared to the TID arm at both 4 and 12 weeks of treatment. The explanation of this response to lower dose iron treatment may be related to hepcidin which is the key regulator of systemic iron balance in the human body, acting in concert with intracellular iron metabolism. Hepcidin inhibits iron absorption in the duodenum and the release of iron from macrophages. Continuous administration of iron replacement will increase the amount of hepcidin in the bloodstream throughout the day. This is inversely proportional to iron bioavailability and impairs transferrin saturation, especially if iron administration is divided in a day [9]. This was in accordance with the previous studies by Moretti D et al. in which after oral iron administration of more than 60 mg of elemental iron either in a divided dose twice daily or once a day, serum hepcidin will increase and affect fractional iron absorption. Lower dosages (40–80 mg) of elemental iron and avoiding twice-daily dosing maximize fractional iron absorption. The duration of the hepcidin response supports the administration of the drug every other day [9]. Stoffel NU et al. also demonstrated that alternate-day dosing of 100 and 200 mg iron in women with IDA sharply increased fractional iron absorption. Even if hepcidin expression is strongly suppressed by iron deficiency and erythropoietic drive, the intake of oral iron leads to an acute hepcidin increase for 24 h [28].
The patients who had symptoms almost fully recovered at week 4, and all were symptom-free at week 12, albeit with different hematological and iron profile responses. This observation was consistent with a previous study by Jimenez et al. [31], who noted that some patients may report an improved sense of well-being a few days after initiating iron treatment.
Serum ferritin level is often used as an indication of iron storage in the body. Normal serum ferritin level ranges from 30 to 400 ng/mL. In IDA, it may take 3 to 6 months after iron replacement therapy to replenish iron storage [12]. Our study also confirmed that serum ferritin continuously increased after iron therapy. Serum ferritin levels in the TIW arm slowly returned to normal at 44.8% and 63.0% after 4 and 12 weeks, respectively. Approximately 73% of the TID arm had serum ferritin in the normal range after 12 weeks of therapy. The proportion of patients who had serum ferritin > 30 ng/mL was not different between the two groups at week 12 (64.3% vs. 73.1%, p = 0.430). Thirty-six percent and 27% in the TIW arm and TID arm, respectively, were still in the iron-deficient state in which serum ferritin was below 30 ng/mL after a 12-week duration of treatment. This may be due to continuous loss such as in the case of menstrual bleeding; on the other hand, it is suggestive of the need for more time to replenish iron storage.
The adverse events of oral iron treatment were predominately gastrointestinal symptoms. More episodes of nausea and epigastric discomfort were reported in the TID arm than in the TIW arm. This was in accordance with the previous studies by Stoffel NU et al. [28], Kaundal R et al. [29], and Mehta S et al. [32] in which AEs in a twice-daily regimen were more than alternate-day regimen, as well as Souza AI et al. [33], in which AEs in a daily regimen was more than twice-day, and weekly, respectively. In addition, it is evident that the higher the dosage of oral iron was, the higher the adverse events were. This may lead to lower adherence to the treatment.
In this investigation, compliance in the two arms was not different at week 4, week 12, and the overall study. The compliance with the twice-day regimen was 98.5%. This contrasted with the previous study by Souza AI et al. [33], who observed that compliance in the weekly regimens was more than twice-weekly and daily regimens. Compliance in both arms was high and similar. The high compliance observed herein was in accord with a similar study by Kulnigg S et al. [34], who reported high compliance (99.2%) among patients who received 200 mg of iron per day for 12 weeks.
The duration of 12-week therapy in our study was longer than the previous low-dose iron treatment in adult IDA studies [29, 32] which is the right time to assess iron store in IDA.
Concerning cost-effectiveness issues especially for developing countries, the TIW regimen reduces pill burden; 18 tablets of ferrous fumarate were saved every week. This will reduce the pill burden by six times and the corresponding cost of receiving medications appropriate to their clinical needs, the proper doses, for an adequate period, and at the lowest cost. [35] Thrice a week of oral iron treatment should be recommended for the treatment of IDA in an adult patient to obtain effective treatment outcomes. It is comparable and non-inferior to traditional oral iron treatment two or three times a day. This insight is important and valuable for informed decision-making and practice of the primary doctor, the iron deficiency anemia treatment guideline committee, and the co-payment government authority.
In conclusion, this study demonstrated that treatment of IDA with oral ferrous fumarate administered thrice weekly (TIW) is not inferior in endpoint therapeutic response to traditional treatment with a thrice-daily regimen at 12-week treatment. In addition, there were fewer adverse events and might enhance patient adherence.
Limitation
During the course of this investigation, we encountered a challenge due to the Coronavirus 2019 pandemic situation. Six patients were unable to visit the study site due to COVID-19 travel restrictions. They were investigated by the physician at the nearby hospital and reported the results to us. Consequently, the laboratory values might be different from the study site. The sample size, based on our hypothesis and non-inferiority margin, was not a big limitation to conclude the non-inferiority between the two regimens.
References
Kassebaum NJ (2016) The global burden of anemia. Hematol Oncol Clin North Am 30(2):247–308. https://doi.org/10.1016/j.hoc.2015.11.002
WHO Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity [Internet]. 2011 World Health Organization. https://www.who.int/vmnis/indicators/haemoglobin.pdf. Accessed 11 May 2022
Daru J, Colman K, Stanworth SJ, De La Salle B, Wood EM, Pasricha SR (2017) Serum ferritin as an indicator of iron status: what do we need to know? Am J Clin Nutr 106:1634s–1639s. https://doi.org/10.3945/ajcn.117.155960
Hallberg L, Bengtsson C, Lapidus L, Lindstedt G, Lundberg PA, Hulten L (1993) Screening for iron deficiency: an analysis based on bone-marrow examinations and serum ferritin determinations in a population sample of women. Br J Haematol 85(4):787–798. https://doi.org/10.1111/j.1365-2141.1993.tb03225.x
Harju E, Pakarinen A, Larmi T (1984) A comparison between serum ferritin concentration and the amount of bone marrow stainable iron. Scand J Clin Lab Invest 44(6):555–556. https://doi.org/10.1080/00365518409083610
Milman N, Bangsboll S, Pedersen NS, Visfeldt J (1983) Serum ferritin in non-dialysis patients with chronic renal failure: relation to bone marrow iron stores. Scand J Haematol 30(4):337–344. https://doi.org/10.1111/j.1600-0609.1983.tb01502.x
Snook J, Bhala N, Beales IL, Cannings D, Kightley C, Logan RP, Pritchard DM, Sidhu R, Surgenor S, Thomas W, Verma AM (2021) British Society of Gastroenterology guidelines for the management of iron deficiency anaemia in adults. Gut 70(11):2030–2051. https://doi.org/10.1136/gutjnl-2021-325210
Tatro DS (2017) Drug interaction handbook, 26th edn. Wolters Kluwer Health, Missouri
Moretti D, Goede JS, Zeder C, Jiskra M, Chatzinakou V, Tjalsma H, Melse-Boonstra A, Brittenham G, Swinkels DW, Zimmermann MB (2015) Oral iron supplements increase hepcidin and decrease iron absorption from daily or twice-daily doses in iron-depleted young women. Blood 126(17):1981–1989. https://doi.org/10.1182/blood-2015-05-642223
Goddard AF, James MW, McIntyre AS, Scott BB, British Society of G (2011) Guidelines for the management of iron deficiency anaemia. Gut 60(10):1309–1316. https://doi.org/10.1136/gut.2010.228874
De Franceschi L, Iolascon A, Taher A, Cappellini MD (2017) Clinical management of iron deficiency anemia in adults: systemic review on advances in diagnosis and treatment. Eur J Intern Med 42:16–23. https://doi.org/10.1016/j.ejim.2017.04.018
Ning S, Zeller MP (2019) Management of iron deficiency. Hematology Am Soc Hematol Educ Program 2019(1):315–322. https://doi.org/10.1182/hematology.2019000034
Killip S, Bennett JM, Chambers MD (2007) Iron deficiency anemia. Am Fam Physician 75(5):671–678
Chaudhari DR, Chopade SS, More VP (2012) A comparative study of the efficacy and tolerability of carbonyl iron and ferrous sulfate in iron deficiency anaemia. Int J Health Sci Res 2(9):47–52
Althunian TA, de Boer A, Groenwold RHH, Klungel OH (2017) Defining the noninferiority margin and analysing noninferiority: an overview. Br J Clin Pharmacol 83(8):1636–1642. https://doi.org/10.1111/bcp.13280
Zhong B (2009) How to calculate sample size in randomized controlled trial? J Thorac Dis 1(1):51–54
Chow S-C, Shao J, Wang H (2003) Sample size calculations in clinical research, 2nd edn. Chapman&Hall/CRC
Fogelholm M, Suominen M, Rita H (1994) Effects of low-dose iron supplementation in women with low serum ferritin concentration. Eur J Clin Nutr 48(10):753–756
Lopes MC, Ferreira LO, Batista FM (1999) Use of daily and weekly ferrous sulfate to treat anemic childbearing-age women. Cad Saude Publica 15(4):799–808. https://doi.org/10.1590/s0102-311x1999000400014
Gilgen D, Mascie-Taylor CG (2001) The effect of weekly iron supplementation on anaemia and on iron deficiency among female tea pluckers in Bangladesh. J Hum Nutr Diet 14(3):185–190. https://doi.org/10.1046/j.1365-277x.2001.00291.x
Siddiqui IA, Jaleel A, Rahman MA (2003) Preventive strategy to control iron deficiency anemia in children and adults. J Pak Med Assoc 53(4):131–133
Olsen A, Nawiri J, Magnussen P, Krarup H, Friis H (2006) Failure of twice-weekly iron supplementation to increase blood haemoglobin and serum ferritin concentrations: results of a randomized controlled trial. Ann Trop Med Parasitol 100:251–263. https://doi.org/10.1179/136485906X91486
Ridwan E, Schultink W, Dillon D, Gross R (1996) Effects of weekly iron supplementation on pregnant Indonesian women are similar to those of daily supplementation. Am J Clin Nutr 63(6):884–890. https://doi.org/10.1093/ajcn/63.6.884
Young MW, Lupafya E, Kapenda E, Bobrow EA. The effectiveness of weekly iron supplementation in pregnant women of rural northern Malawi. Trop Doct 2000;30(2):84-88. https://doi.org/10.1177/004947550003000210
Goonewardene M, Liyanage C, Fernando R (2001) Intermittent oral iron supplementation during pregnancy. Ceylon Med J 46(4):132–135. https://doi.org/10.4038/cmj.v46i4.6440
Haidar J, Omwega AM, Muroki NM, Ayana G (2003) Daily versus weekly iron supplementation and prevention of iron deficiency anaemia in lactating women. East Afr Med J 80(1):11–16. https://doi.org/10.4314/eamj.v80i1.8661
Goonewardene IMR, Senadheera DI. Randomized control trial comparing effectiveness of weekly versus daily antenatal oral iron supplementation in preventing anemia during pregnancy. J Obstet Gynaecol Res 2018;44(3):417-424. https://doi.org/10.1111/jog.13546
Stoffel NU, Zeder C, Brittenham GM, Moretti D, Zimmermann MB (2020) Iron absorption from supplements is greater with alternate day than with consecutive day dosing in iron-deficient anemic women. Haematologica 105(5):1232–1239. https://doi.org/10.3324/haematol.2019.220830
Kaundal R, Bhatia P, Jain A, Jain A, Nampoothiri RV, Mishra K, Jandial A, Goni D, Sandal R, Jindal N, Meshram A (2020) Randomized controlled trial of twice-daily versus alternate-day oral iron therapy in the treatment of iron-deficiency anemia. Ann Hematol 99(1):57–63. https://doi.org/10.1007/s00277-019-03871-z
Okam MM, Koch TA, Tran MH (2017) Iron supplementation, response in iron-deficiency anemia: analysis of five trials. Am J Med 130(8):991.e991–991.e998. https://doi.org/10.1016/j.amjmed.2017.03.045
Jimenez K, Kulnigg-Dabsch S, Gasche C (2015) Management of iron deficiency anemia. Gastroenterol Hepatol 11(4):241–250
Mehta S, Sharma BS, Gulati S, Sharma N, Goyal LK, Mehta S (2020) A Prospective, randomized, interventional study of oral iron supplementation comparing daily dose with alternate day regimen using hepcidin as a biomarker in iron deficiency anemia. J Assoc Physicians India 68(5):39–41
Souza AI, Batista Filho M, Bresani CC, Ferreira LO, Figueiroa JN (2009) Adherence and side effects of three ferrous sulfate treatment regimens on anemic pregnant women in clinical trials. Cad Saude Publica 25(6):1225–1233. https://doi.org/10.1590/s0102-311x2009000600005
Kulnigg S, Stoinov S, Simanenkov V, Dudar LV, Karnafel W, Garcia LC, Sambuelli AM, D'haens G, Gasche C (2008) A novel intravenous iron formulation for treatment of anemia in inflammatory bowel disease: the ferric carboxymaltose (FERINJECT) randomized controlled trial. Am J Gastroenterol 103(5):1182–1192. https://doi.org/10.1111/j.1572-0241.2007.01744.x
World Health Organization (2002) Promoting rational use of medicines : core components. World Health Organization. https://apps.who.int/iris/handle/10665/67438. Accessed 5 May 2022
Acknowledgements
The authors would like to thank all subjects who participated in the study and the hematologist staff at the Hematology Unit, Division of Internal Medicine, Faculty of Medicine, Prince of Songkla University, who contributed to the conduction of the study.
Funding
This study was supported by research funding from The Thai Society of Hematology and the Faculty of Pharmaceutical Sciences, Prince of Songkla University.
Author information
Authors and Affiliations
Contributions
JS, LA, DT, and SW conceived the study and obtained funding; all authors contributed to the design of the trial; JS and LA enrolled patients and conducted the investigation, analyzed and interpreted the data, and wrote the first draft of the manuscript; all authors reviewed, edited, and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Conflict of interest
The authors declare no competing interests.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jongkraijakra, S., Doungngern, T., Sripakdee, W. et al. A randomized controlled trial of thrice-weekly versus thrice-daily oral ferrous fumarate treatment in adult patients with iron-deficiency anemia. Ann Hematol 102, 1333–1340 (2023). https://doi.org/10.1007/s00277-023-05198-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-023-05198-2