Abstract
Subcutaneous daratumumab plus bortezomib/cyclophosphamide/dexamethasone (VCd; D-VCd) improved outcomes versus VCd for patients with newly diagnosed immunoglobulin light-chain (AL) amyloidosis in the phase 3 ANDROMEDA study. We report a subgroup analysis of Asian patients (Japan; Korea; China) from ANDROMEDA. Among 388 randomized patients, 60 were Asian (D-VCd, n = 29; VCd, n = 31). At a median follow-up of 11.4 months, the overall hematologic complete response rate was higher for D-VCd versus VCd (58.6% vs. 9.7%; odds ratio, 13.2; 95% confidence interval [CI], 3.3–53.7; P < 0.0001). Six-month cardiac and renal response rates were higher with D-VCd versus VCd (cardiac, 46.7% vs. 4.8%; P = 0.0036; renal, 57.1% vs. 37.5%; P = 0.4684). Major organ deterioration progression-free survival (MOD-PFS) and major organ deterioration event-free survival (MOD-EFS) were improved with D-VCd versus VCd (MOD-PFS: hazard ratio [HR], 0.21; 95% CI, 0.06–0.75; P = 0.0079; MOD-EFS: HR, 0.16; 95% CI, 0.05–0.54; P = 0.0007). Twelve deaths occurred (D-VCd, n = 3; VCd, n = 9). Twenty-two patients had baseline serologies indicating prior hepatitis B virus (HBV) exposure; no patient experienced HBV reactivation. Although grade 3/4 cytopenia rates were higher than in the global safety population, the safety profile of D-VCd in Asian patients was generally consistent with the global study population, regardless of body weight. These results support D-VCd use in Asian patients with newly diagnosed AL amyloidosis. ClinicalTrials.gov Identifier: NCT03201965.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Immunoglobulin light-chain (AL) amyloidosis is a rare disorder caused by clonal expansion of CD38+ plasma cells that produce immunoglobulin light chains that misfold and aggregate into insoluble amyloid fibrils [1]. Deposition of amyloid fibrils in vital organs, most commonly the heart and kidney, can result in severe and life-threatening organ dysfunction [1]. Approved therapies for AL amyloidosis treatment are lacking and standard of care involves therapies targeting plasma cells that were developed for multiple myeloma (MM); the most commonly used regimen for newly diagnosed patients in Europe is bortezomib/cyclophosphamide/dexamethasone (VCd) [2,3,4]. Although reports on the use of bortezomib-based regimens for AL amyloidosis in Japan, Korea, and China are limited [5,6,7,8,9,10], a study by Shimazaki et al. demonstrated that bortezomib-based therapies are widely used in Japan [11]. Rapid and deep hematologic responses are critical for optimal AL amyloidosis treatment. Although outcomes have improved with the use of novel MM therapies, particularly bortezomib-based therapies, more effective and tolerable therapies are needed [2,3,4, 12].
Daratumumab is a human IgGκ monoclonal antibody targeting CD38 with a direct on-tumor [13,14,15,16] and immunomodulatory [17,18,19] mechanism of action. Based on positive efficacy and safety results from clinical trials, intravenous daratumumab (DARA IV) 16 mg/kg and subcutaneous daratumumab (DARA SC) 1800 mg are approved in many countries as monotherapy and in combination with standard-of-care regimens for newly diagnosed MM and relapsed or refractory MM [20,21,22]. Consistent efficacy and safety with the global study population were seen in Asian patients in the phase 3 POLLUX and ALCYONE studies of DARA IV–containing regimens [23, 24]. In the phase 3 OCTANS and LEPUS studies, which enrolled patients at sites in Asia, efficacy and safety results of DARA IV–containing regimens were also consistent [25, 26]. Additionally, efficacy, pharmacokinetics, and safety of DARA SC in Asian patients in the phase 3 COLUMBA study were consistent with the global study population, regardless of patient body weight [27].
In relapsed or refractory AL amyloidosis, daratumumab has demonstrated an acceptable safety profile and encouraging efficacy in terms of hematologic response rates and improvement in organ function [28,29,30,31,32,33,34]. In the phase 3 ANDROMEDA study, safety and efficacy of DARA SC plus VCd (D-VCd) are being evaluated in patients with newly diagnosed AL amyloidosis. Results from the safety run-in of ANDROMEDA demonstrated that D-VCd was well tolerated [35]. In the primary analysis of the randomized portion of the study, D-VCd resulted in a significantly higher hematologic complete response (CR) rate versus VCd (53.3% vs. 18.1%; P < 0.0001) [36]. Deeper and more rapid hematologic responses with D-VCd versus VCd were associated with delayed major organ deterioration, hematologic progression, or death (major organ deterioration progression-free survival [MOD-PFS]) and improved organ responses at 6 months.
To determine whether the efficacy and safety results of D-VCd in Asian patients with newly diagnosed AL amyloidosis are similar to those observed in the global study population, we performed a post hoc analysis of Asian patients (enrolled at sites in Japan, Korea, and China) from ANDROMEDA.
Patients and methods
Patients
A total of 60 Asian patients (enrolled at sites in Japan [n = 28], Korea [n = 20], or China [n = 12]) from ANDROMEDA (enrollment occurred between May 2018 and August 2019) were included in this analysis. Complete eligibility criteria have been published previously [36]. Briefly, eligible patients were ≥ 18 years of age with a histopathologic diagnosis of systemic AL amyloidosis (≥ 1 involved organ) and measurable hematologic disease with no prior therapy. See Online Resource 1 (Supplementary Methods) for additional details.
Study design and treatment
ANDROMEDA is a randomized, open-label, active-controlled, phase 3 study. Patients were randomized (1:1) to receive VCd with or without DARA SC (daratumumab 1800 mg co-formulated with recombinant human hyaluronidase PH20 [2000 U/mL; ENHANZE® drug delivery technology, Halozyme, Inc., San Diego, CA, USA]). All patients received bortezomib 1.3 mg/m2 subcutaneously, cyclophosphamide 300 mg/m2 orally or intravenously [500 mg maximum weekly dose], and dexamethasone 40 mg orally or intravenously once weekly for 6 cycles of 28 days each. DARA SC was administered by manual injection over approximately 5 min weekly in cycles 1 and 2, every 2 weeks in cycles 3–6, and every 4 weeks thereafter until disease progression, until the start of subsequent therapy, or for a maximum of 24 cycles from the start of the study, whichever occurred first. The median follow-up period was 11.4 months. See Online Resource 1 (Supplementary Methods) for additional details.
Endpoints and assessments
The primary endpoint was overall hematologic CR rate at the time of clinical cutoff, as assessed by the independent review committee that was blinded to treatment assignment. Key secondary endpoints included MOD-PFS, major organ deterioration event-free survival (MOD-EFS), organ response rate [37, 38], organ response rate at 6 months, overall survival, hematologic CR at 6 months, hematologic very good partial response or better (≥ VGPR) rate, time to and duration of hematologic CR, and safety. See the Online Resource 1 (Supplementary Methods) for additional details.
Evaluation and statistical analyses
The intent-to-treat (ITT) population included all randomized patients. The safety population included all patients who received ≥ 1 dose of trial treatment. Between-group difference for overall hematologic CR rate in the ITT population was tested using a stratified Cochran–Mantel–Haenszel test, and corresponding common odds ratios, 95% confidence intervals (CIs), and P values were reported. For Asian patients, the P value was derived from a chi-square test. See the Online Resource 1 (Supplementary Methods) for additional details.
Study oversight
The study was approved by independent ethics committees or institutional review boards at each site and was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonisation Good Clinical Practice guidelines. All patients provided written informed consent. The study design and analyses were devised by the investigators and sponsor, and study data were collected by the investigators and their research teams. Final data analysis and verification of accuracy were conducted by Janssen. Investigators were not restricted by confidentiality agreements and had full access to all data. Writing assistance was funded by Janssen Global Services, LLC. The study was sponsored by Janssen Research & Development, LLC, and was registered at ClinicalTrials.gov (NCT03201965).
Results
Patients and treatments
A total of 388 patients were randomized in ANDROMEDA (D-VCd, n = 195; VCd, n = 193) [36]; 60 (15.5%) patients were included in the Asian cohort analysis (D-VCd, n = 29; VCd, n = 31), including 28 patients from 9 sites in Japan (D-VCd, n = 15; VCd, n = 13), 20 patients from 5 sites in Korea (D-VCd, n = 8; VCd, n = 12), and 12 patients from 4 sites in China (D-VCd, n = 6; VCd, n = 6). Baseline patient demographics and clinical characteristics of the Asian cohort were generally well balanced between arms and consistent with the ITT population (Table 1) [36]. In the Asian cohort, median age was 66 (range, 42–82) years, median body weight was 61.7 (range, 38.0–92.0) kg, and median time since diagnosis was 44 (range, 11–304) days. Only 2 patients (both in the VCd arm) in the Asian cohort had a body weight of > 85 kg. The median baseline difference between involved and uninvolved free light chain was 170 (range, 4–9983) mg/L. Thirty-six patients (60.0%) had ≥ 2 organs involved; 70.0% of patients had heart involvement, and 58.3% had kidney involvement. Most patients (71.7%) were classified as cardiac stage II or higher. In the Asian cohort, a higher percentage of D-VCd patients was classified as cardiac stage I and a lower percentage was classified as cardiac stage II compared with VCd patients. Compared to the ITT population [36], median body weight in the Asian cohort was lower, and the percentage of patients with cardiac stage I was higher.
In the ITT population, 193 and 188 patients in the D-VCd and VCd arms, respectively, received ≥ 1 dose of trial treatment [36]; all patients in the Asian cohort received ≥ 1 treatment dose. At the time of clinical data cutoff for the primary analysis (February 14, 2020), 52 (26.7%) D-VCd patients and 68 (35.2%) VCd patients in the ITT population had discontinued treatment (Table 2) [36]. In the Asian cohort, 4 (13.8%) D-VCd patients and 9 (29.0%) VCd patients had discontinued treatment (Table 2). In the global safety population, median duration of treatment was 9.6 (range, 0.03–21.2) months with D-VCd and 5.3 (range, 0.03–7.3) months with VCd [36], and the median number of cycles received was 11 (range, 1–23) with D-VCd and 6 (range, 1–6) with VCd. In the Asian cohort, median duration of treatment was 9.2 (range, 1.0–21.2) months with D-VCd and 5.3 (range, 0.03–6.1) months with VCd, and median number of cycles received was 11 (range, 2–23) with D-VCd and 6 (range, 1–6) with VCd. In the global safety population, 159 (82.4%) and 121 (64.4%) patients received 6 treatment cycles in the D-VCd and VCd arms, respectively, and in the D-VCd arm, 149 (77.2%) patients continued single-agent DARA SC after completing the first 6 cycles [36]. In the Asian cohort, 25 (86.2%) and 22 (71.0%) patients completed 6 treatment cycles in the D-VCd and VCd arms, respectively, and in the D-VCd arm, all 25 (86.2%) patients continued single-agent DARA SC after completing the first 6 cycles. Consistent with the global safety population [36], the incidence of dose reductions was similar between treatment arms (global safety population: cyclophosphamide, 17.6% vs. 13.8%; bortezomib, 25.9% vs. 19.7%; dexamethasone, 27.5% vs. 27.7%; Asian cohort: cyclophosphamide, 24.1% vs. 32.3%; bortezomib, 24.1% vs. 29.0%; dexamethasone, 17.2% vs. 12.9%). Dose reductions were not permitted for DARA SC.
Efficacy
At a median follow-up of 11.4 (range, 0.03–21.3) months for the ITT population, the overall hematologic CR rate was higher with D-VCd versus VCd in the ITT population (53.3% vs. 18.1%; odds ratio, 5.1; 95% CI, 3.2–8.2; P < 0.0001) [36] and Asian cohort (58.6% vs. 9.7%; odds ratio, 13.2; 95% CI, 3.3–53.7; P < 0.0001; Table 3). Hematologic CR rates at 6 months were consistent with overall hematologic CR rates in the ITT population (D-VCd, 49.7% vs. VCd, 14.0%; odds ratio, 6.1; 95% CI, 3.7–10.0; P < 0.0001) [36] and Asian cohort (D-VCd, 58.6% vs. VCd, 9.7%; odds ratio, 13.2; 95% CI, 3.3–53.7; P < 0.0001). Among patients who achieved hematologic CR, median time to hematologic CR was 1.97 months with D-VCd versus 2.79 months with VCd in the global study population [36] and 1.94 months with D-VCd versus 2.83 months with VCd in the Asian cohort. The rate of hematologic ≥ VGPR and overall hematologic response rate were higher with D-VCd versus VCd in the ITT population (≥ VGPR, 78.5% vs. 49.2%; overall response rate, 91.8% vs. 76.7%) [36] and Asian cohort (≥ VGPR, 93.1% vs. 61.3%; overall response rate, 100.0% vs. 93.5%; Table 3). Among patients who achieved hematologic ≥ VGPR, median time to hematologic ≥ VGPR was 0.56 months with D-VCd versus 0.82 months with VCd in the global study population and 0.53 months with D-VCd versus 0.99 months with VCd in the Asian cohort.
Among those evaluable for cardiac response, the 6-month cardiac response rate was higher with D-VCd versus VCd in the global study population (41.5% vs. 22.2%; P = 0.0029) [36] and Asian cohort (46.7% vs. 4.8%; P = 0.0036; Table 3). Among those evaluable for renal response, the 6-month renal response rate was also higher with D-VCd versus VCd in the global study population (53.0% vs. 23.9%; P < 0.0001) [36] and Asian cohort (57.1% vs. 37.5%; P = 0.4684). Cardiac and renal response rates at 6 months were generally higher with D-VCd regardless of baseline cardiac stage in both the global study population and Asian cohort (Table 3).
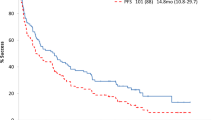
MOD-PFS was improved with D-VCd versus VCd in the ITT population (median: not estimable [NE] in either arm; hazard ratio [HR], 0.57; 95% CI, 0.36–0.91; P = 0.0161; Fig. 1a) and in the Asian cohort (median: NE vs. 13.5 months; HR, 0.21; 95% CI, 0.06–0.75; P = 0.0079; Fig. 1b). In the Asian cohort, 3 events of hematologic progression, major organ deterioration, or death occurred with D-VCd versus 12 events with VCd. MOD-EFS was also improved with D-VCd versus VCd in the ITT population (median, NE vs. 8.8 months; HR, 0.39; 95% CI, 0.27–0.56; P < 0.0001; Fig. 2a) [36] and in the Asian cohort (median, NE vs. 7.4 months; HR, 0.16; 95% CI, 0.05–0.54; P = 0.0007; Fig. 2b). In the Asian cohort, 3 events of hematologic progression, major organ deterioration, initiation of subsequent therapy, or death occurred with D-VCd versus 16 events with VCd. Median time to next treatment was NE with D-VCd versus 10.4 months with VCd in the ITT population (HR, 0.20; 95% CI, 0.12–0.32; P < 0.0001) and NE in either treatment arm in the Asian cohort (HR, 0.10; 95% CI, 0.01–0.79; P = 0.0069). Overall survival results remained immature at the time of this analysis.
MOD-PFSa,b of a the global ITT population and b the Asian cohort. MOD-PFS, major organ deterioration progression-free survival; ITT, intent-to-treat; D-VCd, daratumumab subcutaneous plus bortezomib/cyclophosphamide/dexamethasone; VCd, bortezomib/cyclophosphamide/dexamethasone; NE, not estimable; HR, hazard ratio; CI, confidence interval; IPCW, inverse probability of censoring weighting. aBecause of the small number of Asian patients, an IPCW analysis method was not applicable to analyze MOD-PFS, and MOD-PFS was based on independent review committee assessment after adjusting for dependent censoring due to subsequent non-cross-resistant anti-plasma cell therapy. MOD-PFS was defined as the time from randomization to any of the following events (whichever occurred first): death, clinical manifestation of cardiac or renal failure, or hematologic progression. bEvaluated in the ITT population, which included all randomized patients
MOD-EFSa,b of a the global ITT population and b the Asian cohort. MOD-EFS, major organ deterioration event-free survival; ITT, intent-to-treat; D-VCd, daratumumab subcutaneous plus bortezomib/cyclophosphamide/dexamethasone; VCd, bortezomib/cyclophosphamide/dexamethasone; NE, not estimable; HR, hazard ratio; CI, confidence interval; IPCW, inverse probability of censoring weighting. aBecause of the small number of Asian patients, an IPCW analysis method was not applicable to analyze MOD-EFS, and MOD-EFS was based on independent review committee assessment after adjusting for dependent censoring due to subsequent non-cross-resistant anti-plasma cell therapy. MOD-EFS was defined as hematologic progression, end-stage cardiac or renal disease, initiation of subsequent non-cross-resistant anti-plasma cell therapy, or death, whichever came first. bEvaluated in the ITT population, which included all randomized patients
Efficacy results of the Asian cohort based on baseline body weight are reported in the Supplementary Materials (Online Resource 1 [Supplementary Results] and Online Resource 2 [Supplementary Table 1]).
Safety
Any grade treatment-emergent adverse events (TEAEs) occurred in almost all patients in the global safety population [36] and Asian cohort (Table 4). Grade 3/4 TEAEs occurred in 113 (58.5%) patients with D-VCd and 108 (57.4%) patients with VCd in the global safety population and 19 (65.5%) patients with D-VCd and 25 (80.6%) patients with VCd in the Asian cohort (Table 4). When adjusted for exposure to study treatment, the incidence rate of any grade and grade 3/4 TEAEs was lower with D-VCd versus VCd in the global safety population [36]. In the Asian cohort, the exposure-adjusted incidence rate of grade 3/4 TEAEs was also lower with D-VCd versus VCd, whereas the exposure-adjusted incidence rate of any grade TEAEs was higher with D-VCd versus VCd (Table 4).
The most common any grade (> 25% of patients in any group) and grade 3/4 (≥ 5% of patients in any group) TEAEs are summarized in Table 5. Rates of grade 3/4 lymphopenia (D-VCd, 34.5% and VCd, 32.3%), neutropenia (10.3% and 3.2%), and leukopenia (6.9% and 3.2%) were higher in the Asian cohort compared to the global safety population [36]. In the global safety population, any grade and grade 3/4 infections occurred at a higher rate with D-VCd versus VCd [36]. In the Asian cohort, any grade infections were reported at a similar rate between D-VCd and VCd, while a higher rate of grade 3/4 infections was reported with D-VCd versus VCd (Table 4). In the global safety population, 52 (D-VCd, 25 [13.0%]; VCd, 27 [14.4%]) patients had baseline serologies consistent with prior exposure to hepatitis B virus (HBV). In the Asian cohort, 22 (D-VCd, 10 [34.5%]; VCd, 12 [38.7%]) patients had baseline serologies consistent with prior HBV exposure. No patient in the study had documented HBV reactivation. The rate of grade 3/4 cardiac disorders was similar between D-VCd versus VCd in the global safety population; the rate was lower with D-VCd versus VCd in the Asian cohort (D-VCd, 6.9% and VCd, 12.9%; Table 4). Of the patients who continued to receive single-agent DARA SC, 12 (6.2%) patients in the global safety population [36] and 1 (3.4%) patient in the Asian cohort experienced cardiac disorders from cycle 7 and beyond.
Serious adverse events (SAEs) occurred in 83 (43.0%) patients with D-VCd and 68 (36.2%) patients with VCd in the global safety population [36] and 10 (34.5%) patients with D-VCd and 14 (45.2%) patients with VCd in the Asian cohort (Table 4). The most common SAE was pneumonia in the global safety population (global: D-VCd, 7.3%; VCd, 4.8% [36]; Asian cohort: D-VCd, 0; VCd, 9.7%); cardiac failure (including overall and congestive cardiac failure) was the most common SAE in the Asian cohort (global: D-VCd, 6.2%; VCd, 4.3%; Asian cohort: D-VCd, 10.3%; VCd, 12.9%).
TEAEs leading to treatment discontinuation occurred in 8 patients in each arm in the global safety population (D-VCd, 4.1% and VCd, 4.3%) [36] and 1 patient in each arm of the Asian cohort (3.4% and 3.2%; Table 4). Infections leading to treatment discontinuation of any study treatment occurred in 2 (1.0%) patients with D-VCd and 1 (0.5%) patient with VCd in the global safety population; no infections led to treatment discontinuation in the Asian cohort. TEAEs resulting in death in the global safety population occurred in 22 (11.4%) patients with D-VCd and 15 (8.0%) patients with VCd (Table 4). TEAEs resulting in death in the Asian cohort occurred in 3 (10.3%) patients with D-VCd (cardiac failure [n = 2], sudden death [n = 1]) and 4 (12.9%) patients with VCd (cardiac failure [n = 1], myocardial infarction [n = 1], sinus node dysfunction [n = 1], and ischemic stroke [n = 1]).
In the global safety population, deaths occurred in 27 (14.0%) patients with D-VCd and 29 (15.4%) patients with VCd [36]; deaths during the first 6 months occurred in 25 (13.0%) and 20 (10.6%) patients, respectively. In the Asian cohort, deaths occurred in 3 (10.3%) patients with D-VCd and 9 (29.0%) patients with VCd; deaths during the first 6 months occurred in 3 (10.3%) and 5 (16.1%) patients, respectively. Adverse events were the most common primary cause of death in the global safety population and Asian cohort (global: D-VCd, 11.9%; VCd, 7.4% [36]; Asian cohort: D-VCd, 6.9%; VCd, 9.7%). Disease progression as the primary cause of death was less frequent with D-VCd versus VCd (global: D-VCd, 1.0%; VCd, 4.8% [36]; Asian cohort: D-VCd, 3.4%; VCd, 9.7%), as were other reasons (global: D-VCd, 1.0%; VCd, 2.7% [36]; Asian cohort: D-VCd, 0%; VCd, 9.7%).
Fourteen (7.3%) patients in the global safety population and 3 (10.3%) patients in the Asian cohort experienced systemic administration-related reactions to DARA SC, all of which were grade 1 or 2 [36]. In the global safety population, 54 (28.0%) patients in the D-VCd arm and 45 (23.9%) patients in the VCd arm experienced local injection-site reactions; 21 (10.9%) patients in the D-VCd arm experienced local injection-site reactions related to DARA SC, all of which were grade 1 or 2 [36]. No Asian patient experienced local injection-site reactions.
Safety results of the Asian cohort based on baseline body weight are reported in the Supplementary Materials (Online Resource 1 [Supplementary Results] and Online Resources 3–5 [Supplementary Tables 2–4]).
Discussion
In this post hoc subgroup analysis of Asian patients enrolled in ANDROMEDA, a higher hematologic CR rate and deeper and more rapid hematologic responses were observed with D-VCd versus VCd; results were generally consistent across body weight subgroups. Improved MOD-PFS, MOD-EFS, and cardiac and renal response rates at 6 months were also observed in the Asian cohort, with improved MOD-PFS and 6-month organ response rates seen across body weight subgroups. In the Asian cohort, cardiac and renal response rates at 6 months were generally higher with D-VCd versus VCd, regardless of baseline cardiac stage. These results indicate that the addition of daratumumab to VCd elicits deeper responses and prolongs MOD-PFS and MOD-EFS compared with VCd alone in Asian patients. The efficacy results presented here for the Asian cohort overall and by baseline body weight are consistent with those from the global ANDROMEDA population [36].
Of note, although the hematologic ORR and ≥ VGPR rates in the VCd arm of the ANDROMEDA Asian cohort (ORR, 93.5%; ≥ VGPR, 61.3%) were in line with those from other published reports for bortezomib-containing regimens in Asian patients (ORR, 66.2–90.0%; ≥ VGPR, 54.2–75.0%), a lower proportion of patients in the ANDROMEDA Asian cohort achieved hematologic CR with VCd (CR, 9.7%) compared to these other reports (CR, 36.1–60.0%) [6, 39, 40]. However, such cross-study comparisons should be interpreted with caution due to differences in study designs, treatment regimens, and patient populations.
D-VCd demonstrated an acceptable safety profile in Asian patients that was generally consistent with the global safety population from ANDROMEDA and the known safety profile of the individual components [20, 21, 36, 41]. Consistent with the Asian subgroup analysis of COLUMBA, higher rates of grade 3/4 cytopenias were observed in the Asian cohort of ANDROMEDA versus the global safety population [27, 36]; in the current study, rates were similar between treatment arms. Higher rates of grade 3/4 cytopenias in the Asian cohort may be attributed to lower median baseline body weight in this cohort versus the global safety population. Notably, despite higher rates of grade 3/4 cytopenias in the Asian cohort, rates of grade 3/4 and serious infections in the Asian cohort were similar to or lower than those in the global safety population [36]. Rates of grade 3/4 infections were higher with D-VCd versus VCd in both the global safety population [36] and Asian cohort, which may be attributed to the longer treatment duration and longer adverse event collection period in the D-VCd arm. When adjusted for exposure to study treatment, incidence rates of grade 3/4 TEAEs were lower with D-VCd versus VCd in the global safety population [36] and in the Asian cohort overall and across body weight subgroups. The rate of serious pneumonia, a common SAE associated with daratumumab [20,21,22], was similar between the Asian cohort and global safety population [36], and no patient in the Asian cohort experienced serious pneumonia with D-VCd. Although 36.7% of Asian patients had baseline serologies consistent with prior HBV exposure, in this study, no Asian patient had documented HBV reactivation; these findings were consistent with observations in the global safety population. Patients in the Asian cohort did not experience an increased rate of grade 3/4 cardiac disorders compared to the global safety population, and rates of grade 3/4 cardiac disorders in the Asian cohort were lower with D-VCd, including in the lower body weight subgroup. The rate of TEAEs resulting in death was higher with D-VCd versus VCd in the global safety population but was similar between treatment arms in the Asian cohort. Consistent with the global safety population [36], administration-related reactions were infrequent and mild in the Asian cohort. No local injection-site reactions related to DARA SC were observed in Asian patients.
There are several limitations of this post hoc analysis. The imbalance in cardiac stage between treatment groups in the Asian cohort may have impacted the magnitude of the efficacy differences observed between D-VCd and VCd in favor of the D-VCd group. Additionally, this analysis was limited by data immaturity and by the relatively low patient numbers in the Asian cohort.
Results of this subgroup analysis complement those reported for the Asian subgroup analysis of COLUMBA in relapsed or refractory MM [27]. In Asian patients in COLUMBA, DARA SC 1800 mg flat dose was comparable to DARA IV 16 mg/kg, and no new safety concerns were observed. Efficacy and safety results with DARA SC were consistent with those observed in the global COLUMBA population, regardless of patient body weight [27].
The addition of DARA SC to VCd was superior to VCd alone in Asian patients, resulting in deeper and more rapid hematologic responses and improved organ responses. Treatment with D-VCd improved clinical outcomes, including MOD-PFS and MOD-EFS, versus VCd alone in Asian patients. Although this post hoc subgroup analysis was limited by data immaturity and a small sample size, efficacy and safety of D-VCd in Asian patients overall and of low body weight were generally consistent with those of the global ANDROMEDA population [36]. These results support the use of D-VCd in Asian patients with newly diagnosed AL amyloidosis.
Data availability
The data sharing policy of Janssen Pharmaceutical Companies of Johnson & Johnson is available at https://www.janssen.com/clinical-trials/transparency. As noted on this site, requests for access to the study data can be submitted through the Yale Open Data Access (YODA) Project site at http://yoda.yale.edu.
References
Merlini G, Dispenzieri A, Sanchorawala V, Schonland SO, Palladini G, Hawkins PN, Gertz MA (2018) Systemic immunoglobulin light chain amyloidosis. Nat Rev Dis Primers 4:38. https://doi.org/10.1038/s41572-018-0034-3
Kastritis E, Leleu X, Arnulf B, Zamagni E, Cibeira MT, Kwok F, Mollee P, Hajek R, Moreau P, Jaccard A, Schonland SO, Filshie R, Nicolas-Virelizier E, Augustson B, Mateos MV, Wechalekar A, Hachulla E, Milani P, Dimopoulos MA, Fermand JP, Foli A, Gavriatopoulou M, Klersy C, Palumbo A, Sonneveld P, Johnsen HE, Merlini G, Palladini G (2020) Bortezomib, melphalan, and dexamethasone for light-chain amyloidosis. J Clin Oncol 38:3252–3260. https://doi.org/10.1200/JCO.20.01285
Palladini G, Sachchithanantham S, Milani P, Gillmore J, Foli A, Lachmann H, Basset M, Hawkins P, Merlini G, Wechalekar AD (2015) A European collaborative study of cyclophosphamide, bortezomib, and dexamethasone in upfront treatment of systemic AL amyloidosis. Blood 126:612–615. https://doi.org/10.1182/blood-2015-01-620302
Manwani R, Cohen O, Sharpley F, Mahmood S, Sachchithanantham S, Foard D, Lachmann HJ, Quarta C, Fontana M, Gillmore JD, Whelan C, Hawkins PN, Wechalekar AD (2019) A prospective observational study of 915 patients with systemic AL amyloidosis treated with upfront bortezomib. Blood 134:2271–2280. https://doi.org/10.1182/blood.2019000834
Huang X, Wang Q, Chen W, Zeng C, Chen Z, Gong D, Zhang H, Liu Z (2014) Induction therapy with bortezomib and dexamethasone followed by autologous stem cell transplantation versus autologous stem cell transplantation alone in the treatment of renal AL amyloidosis: a randomized controlled trial. BMC Med 12:2. https://doi.org/10.1186/1741-7015-12-2
Katoh N, Ueno A, Yoshida T, Tazawa KI, Shimojima Y, Gono T, Sekijima Y, Matsuda M, Ikeda SI (2017) Bortezomib-dexamethasone versus high-dose melphalan for Japanese patients with systemic light-chain (AL) amyloidosis: a retrospective single-center study. Int J Hematol 105:341–348. https://doi.org/10.1007/s12185-016-2128-6
Shimazaki C, Fuchida S, Suzuki K, Ishida T, Imai H, Sawamura M, Takamatsu H, Abe M, Miyamoto T, Hata H, Yamada M, Ando Y (2016) Phase 1 study of bortezomib in combination with melphalan and dexamethasone in Japanese patients with relapsed AL amyloidosis. Int J Hematol 103:79–85. https://doi.org/10.1007/s12185-015-1901-2
Hur JY, Lee KK, Yoon SE, Park S, Cho J, Kim Y, Jeon E, Choi J, Lee G, Kim B, Min JH, Kim JS, Lee JE, Choi JY, Kim SJ, Jang JH, Kim WS, Jung CW, Kim K (2018) Bortezomib-based first line treatment for AL amyloidosis patients who are not candidate for stem cell transplantation. Blood 132:3256. https://doi.org/10.1182/blood-2018-99-114099
Shen KN, Zhang CL, Tian Z, Feng J, Wang YN, Sun J, Zhang L, Cao XX, Zhou DB, Li J (2019) Bortezomib-based chemotherapy reduces early mortality and improves outcomes in patients with ultra-high-risk light-chain amyloidosis: a retrospective case control study. Amyloid 26:66–73. https://doi.org/10.1080/13506129.2019.1594759
Huang X, Wang Q, Chen W, Ren G, Liu Z (2016) Bortezomib with dexamethasone as first-line treatment for AL amyloidosis with renal involvement. Amyloid 23:51–57. https://doi.org/10.3109/13506129.2016.1138939
Shimazaki C, Hata H, Iida S, Ueda M, Katoh N, Sekijima Y, Ikeda S, Yazaki M, Fukushima W, Ando Y (2018) Nationwide survey of 741 patients with systemic amyloid light-chain amyloidosis in Japan. Intern Med 57:181–187. https://doi.org/10.2169/internalmedicine.9206-17
Muchtar E, Gertz MA, Kumar SK, Lacy MQ, Dingli D, Buadi FK, Grogan M, Hayman SR, Kapoor P, Leung N, Fonder A, Hobbs M, Hwa YL, Gonsalves W, Warsame R, Kourelis TV, Russell S, Lust JA, Lin Y, Go RS, Zeldenrust S, Kyle RA, Rajkumar SV, Dispenzieri A (2017) Improved outcomes for newly diagnosed AL amyloidosis between 2000 and 2014: cracking the glass ceiling of early death. Blood 129:2111–2119. https://doi.org/10.1182/blood-2016-11-751628
de Weers M, Tai YT, van der Veer MS, Bakker JM, Vink T, Jacobs DC, Oomen LA, Peipp M, Valerius T, Slootstra JW, Mutis T, Bleeker WK, Anderson KC, Lokhorst HM, van de Winkel JG, Parren PW (2011) Daratumumab, a novel therapeutic human CD38 monoclonal antibody, induces killing of multiple myeloma and other hematological tumors. J Immunol 186:1840–1848. https://doi.org/10.4049/jimmunol.1003032
Overdijk MB, Jansen JH, Nederend M, Lammerts van Bueren JJ, Groen RW, Parren PW, Leusen JH, Boross P (2016) The therapeutic CD38 monoclonal antibody daratumumab induces programmed cell death via Fcγ receptor-mediated cross-linking. J Immunol 197:807–813. https://doi.org/10.4049/jimmunol.1501351
Overdijk MB, Verploegen S, Bogels M, van Egmond M, Lammerts van Bueren JJ, Mutis T, Groen RW, Breij E, Martens AC, Bleeker WK, Parren PW (2015) Antibody-mediated phagocytosis contributes to the anti-tumor activity of the therapeutic antibody daratumumab in lymphoma and multiple myeloma. MAbs 7:311–321. https://doi.org/10.1080/19420862.2015.1007813
Lammerts van Bueren J, Jakobs D, Kaldenhoven N, Roza M, Hiddingh S, Meesters J, Voorhorst M, Gresnigt E, Wiegman L, Buijsse O, Andringa G, Overdijk MB, Doshi P, Sasser K, de Weers M, Parren PWHI (2014) Direct in vitro comparison of daratumumab with surrogate analogs of CD38 antibodies MOR03087, SAR650984 and Ab79. Blood 124:3474. https://doi.org/10.1182/blood.V124.21.3474.3474
Krejcik J, Casneuf T, Nijhof IS, Verbist B, Bald J, Plesner T, Syed K, Liu K, van de Donk NWCJ, Weiss BM, Ahmadi T, Lokhorst HM, Mutis T, Sasser AK (2016) Daratumumab depletes CD38+ immune-regulatory cells, promotes T-cell expansion, and skews T-cell repertoire in multiple myeloma. Blood 128:384–394. https://doi.org/10.1182/blood-2015-12-687749
Adams HC III, Stevenaert F, Krejcik J, Van der Borght K, Smets T, Bald J, Abraham Y, Ceulemans H, Chiu C, Vanhoof G, Usmani SZ, Plesner T, Lonial S, Nijhof I, Lokhorst HM, Mutis T, van de Donk N, Sasser AK, Casneuf T (2019) High-parameter mass cytometry evaluation of relapsed/refractory multiple myeloma patients treated with daratumumab demonstrates immune modulation as a novel mechanism of action. Cytometry A 95:279–289. https://doi.org/10.1002/cyto.a.23693
Casneuf T, Adams HC III, van de Donk N, Abraham Y, Bald J, Vanhoof G, Van der Borght K, Smets T, Foulk B, Nielsen KC, Rusbuldt J, Axel A, Lysaght A, Ceulemans H, Stevenaert F, Usmani SZ, Plesner T, Avet-Loiseau H, Nijhof I, Mutis T, Schecter JM, Chiu C, Bahlis NJ (2021) Deep immune profiling of patients treated with lenalidomide and dexamethasone with or without daratumumab. Leukemia 35:573–584. https://doi.org/10.1038/s41375-020-0855-4
DARZALEX® (2022) (daratumumab) injection, for intravenous use [package insert]. Horsham, PA: Janssen Biotech, Inc
DARZALEX FASPRO™ (2022) (daratumumab and hyaluronidase-fihj) [package insert]. Horsham, PA: Janssen Biotech, Inc
European Medicines Agency (2022) Darzalex 20 mg/mL concentrate for solution for infusion. Summary of product characteristics. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/004077/WC500207296.pdf. Accessed 15 Feb 2023
Fujisaki T, Ishikawa T, Takamatsu H, Suzuki K, Min CK, Lee JH, Wang J, Carson R, Crist W, Qi M, Nagafuji K (2019) Daratumumab plus bortezomib, melphalan, and prednisone in East Asian patients with non-transplant multiple myeloma: subanalysis of the randomized phase 3 ALCYONE trial. Ann Hematol 98:2805–2814. https://doi.org/10.1007/s00277-019-03794-9
Suzuki K, Dimopoulos M, Takezako N, Okamoto S, Shinagawa A, Matsumoto M, Kosugi H, Yoon S, Huang S, Qin X, Qi M, Iida S (2018) Daratumumab, lenalidomide, and dexamethasone in East Asian patients with relapsed or refractory multiple myeloma: subgroup analyses of the phase 3 POLLUX study. Blood Cancer J 8:41. https://doi.org/10.1038/s41408-018-0071-x
Hou J, Fu W, Bang SM, Huang H, Kim K, Li W, An G, Lee JJ, Cai Z, Jin J, Wang Y, Chim CS, Qi M, Wang J, Lu X, Song Y, Jia B, Yang X, Liu W, Li Y, Zhang R, Wang J (2021) EP1024 Phase 3 study of daratumumab, bortezomib, melphalan, and prednisone (D-VMP) versus bortezomib, melphalan, and prednisone (VMP) in Asian patients with newly diagnosed multiple myeloma (NDMM): OCTANS. HemaSphere 5:e566. https://doi.org/10.1097/HS9.0000000000000566
Lu J, Fu W, Li W, Hu J, An G, Wang Y, Fu C, Chen L, Jin J, Cen X, Ge Z, Cai Z, Niu T, Qi M, Sun S, Gai X, Liu W, Liu W, Yang X, Huang X (2021) Daratumumab, bortezomib, and dexamethasone versus bortezomib and dexamethasone in Chinese patients with relapsed or refractory multiple myeloma: phase 3 LEPUS (MMY3009) study. Clin Lymphoma Myeloma Leuk 21:e699–e709. https://doi.org/10.1016/j.clml.2021.04.012
Iida S, Ishikawa T, Min CK, Kim K, Yeh SP, Usmani SZ, Mateos MV, Nahi H, Heuck C, Qin X, Parasrampuria DA, Gries KS, Qi M, Bahlis N, Ito S (2021) Subcutaneous daratumumab in Asian patients with heavily pretreated multiple myeloma: subgroup analyses of the noninferiority, phase 3 COLUMBA study. Ann Hematol 100:1065–1077. https://doi.org/10.1007/s00277-021-04405-2
Kaufman GP, Schrier SL, Lafayette RA, Arai S, Witteles RM, Liedtke M (2017) Daratumumab yields rapid and deep hematologic responses in patients with heavily pretreated AL amyloidosis. Blood 130:900–902. https://doi.org/10.1182/blood-2017-01-763599
Khouri J, Kin A, Thapa B, Reu FJ, Bumma N, Samaras CJ, Liu HD, Karam MA, Reed J, Mathur S, Faiman BM, Devries G, Zonder J, Valent J (2019) Daratumumab proves safe and highly effective in AL amyloidosis. Br J Haematol 185:342–344. https://doi.org/10.1111/bjh.15455
Abeykoon JP, Zanwar S, Dispenzieri A, Gertz MA, Leung N, Kourelis T, Gonsalves W, Muchtar E, Dingli D, Lacy MQ, Hayman SR, Buadi F, Warsame R, Kyle RA, Rajkumar V, Kumar S, Kapoor P (2019) Daratumumab-based therapy in patients with heavily-pretreated AL amyloidosis. Leukemia 33:531–536. https://doi.org/10.1038/s41375-018-0262-2
Roussel M, Merlini G, Chevret S, Arnulf B, Stoppa AM, Perrot A, Palladini G, Karlin L, Royer B, Huart A, Macro M, Morel P, Frenzel L, Touzeau C, Boyle E, Dorvaux V, Le Bras F, Lavergne D, Bridoux F, Jaccard A (2020) A prospective phase 2 trial of daratumumab in patients with previously treated systemic light-chain amyloidosis. Blood 135:1531–1540. https://doi.org/10.1182/blood.2019004369
Sanchorawala V, Sarosiek S, Schulman A, Mistark M, Migre ME, Cruz R, Sloan JM, Brauneis D, Shelton AC (2020) Safety, tolerability, and response rates of daratumumab in relapsed AL amyloidosis: results of a phase 2 study. Blood 135:1541–1547. https://doi.org/10.1182/blood.2019004436
Kimmich CR, Terzer T, Benner A, Dittrich T, Veelken K, Carpinteiro A, Hansen T, Goldschmidt H, Seckinger A, Hose D, Jauch A, Worner S, Beimler J, Muller-Tidow C, Hegenbart U, Schonland SO (2020) Daratumumab for systemic AL amyloidosis: prognostic factors and adverse outcome with nephrotic-range albuminuria. Blood 135:1517–1530. https://doi.org/10.1182/blood.2019003633
Milani P, Fazio F, Basset M, Berno T, Larocca A, Foli A, Riva M, Benigna F, Oliva S, Nuvolone M, Rodigari L, Petrucci MT, Merlini G, Palladini G (2020) High rate of profound clonal and renal responses with daratumumab treatment in heavily pre-treated patients with light chain (AL) amyloidosis and high bone marrow plasma cell infiltrate. Am J Hematol 95:900–905. https://doi.org/10.1002/ajh.25828
Palladini G, Kastritis E, Maurer MS, Zonder JA, Minnema MC, Wechalekar AD, Jaccard A, Lee HC, Bumma N, Kaufman JL, Medvedova E, Kovacsovics TJ, Rosenzweig MA, Sanchorawala V, Qin X, Vasey SY, Weiss B, Vermeulen J, Merlini G, Comenzo RL (2020) Daratumumab plus CyBorD for patients with newly diagnosed AL amyloidosis: safety run-in results of ANDROMEDA. Blood 136:71–80. https://doi.org/10.1182/blood.2019004460
Kastritis E, Palladini G, Minnema MC, Wechalekar AD, Jaccard A, Lee HC, Sanchorawala V, Gibbs S, Mollee P, Venner CP, Lu J, Schönland S, Gatt ME, Suzuki K, Kim K, Cibeira MT, Beksac M, Libby E, Valent J, Hungria V, Wong SW, Rosenzweig M, Bumma N, Huart A, Dimopoulos MA, Bhutani D, Waxman AJ, Goodman SA, Zonder JA, Lam S, Song K, Hansen T, Manier S, Roeloffzen W, Jamroziak K, Kwok F, Shimazaki C, Kim JS, Crusoe E, Ahmadi T, Tran NP, Qin X, Vasey SY, Tromp B, Schecter JM, Weiss BM, Zhuang SH, Vermeulen J, Merlini G, Comenzo RL (2021) Daratumumab-based treatment for immunoglobulin light-chain amyloidosis. N Engl J Med 385:46–58. https://doi.org/10.1056/NEJMoa2028631
Palladini G, Dispenzieri A, Gertz MA, Kumar S, Wechalekar A, Hawkins PN, Schonland S, Hegenbart U, Comenzo R, Kastritis E, Dimopoulos MA, Jaccard A, Klersy C, Merlini G (2012) New criteria for response to treatment in immunoglobulin light chain amyloidosis based on free light chain measurement and cardiac biomarkers: impact on survival outcomes. J Clin Oncol 30:4541–4549. https://doi.org/10.1200/JCO.2011.37.7614
Palladini G, Hegenbart U, Milani P, Kimmich C, Foli A, Ho AD, Vidus Rosin M, Albertini R, Moratti R, Merlini G, Schonland S (2014) A staging system for renal outcome and early markers of renal response to chemotherapy in AL amyloidosis. Blood 124:2325–2332. https://doi.org/10.1182/blood-2014-04-570010
Feng J, Zhang C, Shen K, Sun J, Fang Q, Zhang L, Cao X, Zhou D, Li J, Tian Z (2019) Outcome of cardiac light-chain amyloidosis in the era of novel therapy—a single-center cohort study of 227 patients. Circ J 83:775–782. https://doi.org/10.1253/circj.CJ-18-1048
Shen KN, Fu WJ, Wu Y, Dong YJ, Huang ZX, Wei YQ, Li CR, Sun CY, Chen Y, Miao HL, Zhang YL, Cao XX, Zhou DB, Li J (2022) Doxycycline combined with bortezomib-cyclophosphamide-dexamethasone chemotherapy for newly diagnosed cardiac light-chain amyloidosis: a multicenter randomized controlled trial. Circulation 145:8–17. https://doi.org/10.1161/CIRCULATIONAHA.121.055953
Jimenez Zepeda VH, Duggan P, Neri PE, Bahlis NJ (2014) Cyclophosphamide, bortezomib and dexamethasone (CyBORD) is a feasible and active regimen for non-transplant eligible multiple myeloma patients. Blood 124:5751. https://doi.org/10.1182/blood.V124.21.5751.5751
Muchtar E, Dispenzieri A, Leung N, Lacy MQ, Buadi FK, Dingli D, Hayman SR, Kapoor P, Hwa YL, Fonder A, Hobbs M, Gonsalves W, Kourelis TV, Warsame R, Russell SJ, Lust JA, Lin Y, Go RS, Zeldenrust SR, Kyle RA, Rajkumar SV, Kumar SK, Gertz MA (2019) Optimizing deep response assessment for AL amyloidosis using involved free light chain level at end of therapy: failure of the serum free light chain ratio. Leukemia 33:527–531. https://doi.org/10.1038/s41375-018-0258-y
Acknowledgements
The authors would like to acknowledge the patients participating in this study and their families, the staff members at the study sites, the data and safety monitoring committees, and the staff members who were involved in data collection and analyses.
Funding
This study was sponsored by Janssen Research & Development, LLC. Medical writing and editorial support were provided by Grace Wang, PharmD, of Lumanity Communications Inc., and funded by Janssen Global Services, LLC.
Author information
Authors and Affiliations
Contributions
All authors drafted and reviewed the paper, approved the final version, decided to publish this report, and vouch for data accuracy and completeness.
Corresponding author
Ethics declarations
Ethics approval
An independent ethics committee or institutional review board approved the trial. The study protocol was conducted in accordance with the principals of the Declaration of Helsinki and the International Conference on Harmonisation guidelines on Good Clinical Practice.
Consent to participate
All patients provided written informed consent.
Competing interests
KS consulted for and received honoraria and research funding from Celgene and Amgen; consulted for and received honoraria from Takeda and Janssen; received honoraria and research funding from Bristol Myers Squibb; and received honoraria from Ono, Novartis, Sanofi, and AbbVie. ADW received honoraria from and served on a board of directors or advisory committees for Janssen, Takeda, Caelum, and Celgene. KK consulted for and received honoraria and research funding from Bristol Myers Squibb, Takeda, Amgen, Celgene, and Janssen. CS received honoraria from Sanofi, Bristol Myers Squibb, and Janssen. FZ is currently employed by Peking University First Hospital. SI received honoraria and research funding from Sanofi, Bristol Myers Squibb, Daiichi Sankyo, Takeda, Ono, Celgene, and Janssen; and received research funding from Merck, AbbVie, Kyowa Kirin, and Chugai. NK received honoraria from Janssen. NT, SYV, and BT are current employees of Janssen and hold equity in Johnson & Johnson. XQ is a current employee of Janssen. BMW was an employee of Janssen at the time of the study. RLC consulted for and received research funding from Prothena, Janssen, Takeda, and Karyopharm and consulted for Amgen, Sanofi, Unum, and Caelum. EK consulted for and received honoraria and research funding from Janssen and Amgen; consulted for and received honoraria from Genesis and Takeda; was reimbursed by Janssen, Genesis, and Takeda for travel, accommodations, and expenses; and consulted for Pfizer. JSK, TI, C-KM, ZC, XC, TF, H-JS, and JL had no relevant conflicts of interest to disclose.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Suzuki, K., Wechalekar, A.D., Kim, K. et al. Daratumumab plus bortezomib, cyclophosphamide, and dexamethasone in Asian patients with newly diagnosed AL amyloidosis: subgroup analysis of ANDROMEDA. Ann Hematol 102, 863–876 (2023). https://doi.org/10.1007/s00277-023-05090-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-023-05090-z